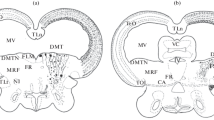
The aim of the present work was to study the expression of intermediate filament proteins – vimentin and glial fibrillary acidic protein (GFAP) – in neocortical cells in rat embryos at different time points after allotransplantation into the lesioned peripheral sciatic nerve of adult animals. Immunohistochemical studies followed the formation of GFAP-positive astrocytes from vimentin-positive radial gliocytes in the neocortex rudiments of rat embryos developing in the damaged nerve. In these conditions, the formation of astrocytes by the embryo neocortex was found to occur several days earlier than in the rat neocortex in normal ontogeny. Transplants surviving for prolonged periods showed the development of reactive gliosis, as evidenced by the presence of large numbers of intensely staining GFAP-positive cells and vimentincontaining astrocytes. These data provide evidence that ectopic neural transplantation provides a model for basic studies of the mechanisms of development of reactive gliosis.
Similar content being viewed by others
References
M. A. Aleksandrova, Methods of Differentiation of Nervous tissue and Intercellular Interactions on Neural Transplantation in Mammals [in Russian], Author’s Abstract of Doctoral Thesis in Biological Sciences, Moscow (1999).
O. S. Vinogradova, “Methods of cultivating nervous tissue in vivo,” in: Handbook on the Cultivation of Nervous Tissue [in Russian], Nauka, Moscow (1988), pp. 49–66.
A. V. Gilyarov, Nestin in Rat Brain Cells (an immunohistochemical study) [in Russian], Author’s Abstract of Master’s Thesis in Medical Sciences, St. Petersburg (2008).
D. E. Korzhevskii and A. V. Gilyarov, Basic Histological Techniques [in Russian], SpetsLit, St. Petersburg (2010).
D. E. Korzhevskii, M. I. Lentsman, O. V. Kirik, and V. A. Otellin, “Vimentin-immunopositive cells in the telencephalon in rats after experimental ischemic stroke,” Morfologiya, 132, No. 5, 23–27 (2007).
V. A. Otellin and E. S. Petrova, “Structure of long-lived transplants of rat embryo CNS rudiments,” Morfologiya, 113, No. 2, 39–44 (1998).
E. S. Petrova, Development of Rat Embryo CNS Rudiments in a Damaged Peripheral Nerve [in Russian], Author’s Abstract of Master’s Thesis in Biological Sciences, St. Petersburg (1991).
E. S. Petrova and V. A. Otellin, “Characteristics of development of homo- and heterotopic allotransplants of the rat embryo neocortex,” Tsitologiya, 42, No. 8, 750–757 (2000).
E. S. Petrova and V. A. Otellin, “Dystrophic changes and cell death in long-lived homo- and heterotopic transplants of rat embryo neocortex rudiments” Byull. Eksperim. Biol., 136, No. 9, 343–347 (2003).
E. I. Chumasov and E. S. Petrova, “Implantation of embryonic neocortex and spinal cord rudiments in a damaged peripheral nerve in adult rats,” Byull. Eksperim. Biol., 108, No. 8, 198–201 (1990).
Y. Amoh, L. Li, K. Campillo, et al., “Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves,” Proc. Natl. Acad. Sci. USA, 102, No. 49, 17734–17738 (2005).
J. J. Bernstein, “Viability, growth and maturation of fetal brain and spinal cord in the sciatic nerve of adult rat,” J. Neurosci. Res., 10, No. 4, 343–350 (1983).
B. H. Choi, “Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study,” J. Neuropathol. Exp. Neurol., 45, No. 4, 408–418 (1986).
L. C. Doering and A. J. Aguayo, “Hirano bodies and other cytoskeletal abnormalities develop in fetal rat CNS grafts isolated for long periods in peripheral nerve,” Brain Res., 401, No. 1, 178–184 (1987).
L. F. Eng, R. S. Ghirnikar, and Y. L. Lee, “Glial fibrillary acidic protein: GFAP thirty-one years (1969–2000),” Neurochem. Res., 25, No. 9, 1439–1451 (2000).
B. Juliandi, M. Abematsu, and K. Nakashima, “Epigenetic regulation in neural stem cell differentiation,” Dev. Growth Differ., 52, No. 6, 493–504 (2010).
P. Levitt and P. Rakic, “Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing Rhesus monkey brain,” J. Comp. Neurol., 193, 815–840 (1980).
X. Nie, Y.-J. Zhang, W. D. Tian, et al., “Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells,” Int. J. Oral Maxillofac. Surg., 36, No. 1, 32–38 (2007).
S. K. Pixley and J. de Vellis, “Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin,” Brain Res., 317, No. 2, 201–209 (1984).
P. M. Richardson and V. W. K. Issa, “Transplantation of embryonic spinal and cerebral tissue to sciatic nerves of adult rats,” Brain Res., 298, No. 1, 146–148 (1984).
R. Trivedi, R. K. Gupta, N. Husain, et al., “Region-specific maturation of cerebral cortex in human fetal brain: diffusion tensor imaging and histology,” Neuroradiology, 51, No. 9, 567–576 (2009).
A. Tuba, L. Kallai, and M. Kalman, “A rapid replacement of vimentin-containing radial glia by glial fibrillary acidic protein-containing astrocytes in transplanted telencephalon,” J. Neural Transplant. Plast., 6, No. 1, 21–29 (1997).
T. Voight, “Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes,” J. Comp. Neurol., 289, No. 1, 74–88 (1989).
S. Wen, H. Li, and J. Liu, “Epigenetic background of neuronal fate determination,” Prog. Neurobiol., 87, No. 2, 98–117 (2009).
A. Widestand, J. Faijerson, U. Wilhelmsson, et al., “Increased neurogenesis and astrogenesis form neuronal progenitor cells grafted in the hippocampus of GFAP–/–Vim–/– mice,” Stem Cells, 10, 2619–2627 (2007).
G. Xiong, N. Ozaki, and Y. Sugiura, “Transplanted embryonic spinal tissues promotes severed sciatic nerve regeneration in rats,” Arch. Histol. Cytol., 72, No. 2, 127–138 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Morfologiya, Vol. 139, No. 2, pp. 22–26, March–April, 2011.
Rights and permissions
About this article
Cite this article
Petrova, E.S. Vimentin and Glial Fibrillary Acidic Protein in the Cells of Ectopic Neural Transplants of Rat Neocortex. Neurosci Behav Physi 42, 598–602 (2012). https://doi.org/10.1007/s11055-012-9607-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-012-9607-x