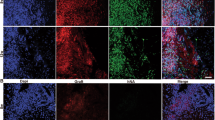
We studied individual peculiarities of the development and differentiation of allogeneic transplants of neocortical cells isolated from embryos at different stages of development in intact brain of adult mice. Despite standard transplantation technique, intraparenchymal grafts considerably varied in size, morphology, and structural organization. The cells in the transplants developing inside the brain ventricles of the recipient formed histotypical structures resembling organoids. Transplants of each age group (12.5, 14.5, and 19.5 days) demonstrated individual peculiarities of cell migration, differentiation, and fiber growth. Only from cells of 12.5-day transplants formed spiny pyramidal neurons typical of V layer of the cerebral cortex. Differentiation of catecholaminergic neurons untypical of brain cortex was observed only in 14.5-day transplants. In few transplants of each age group, extensive cell migration from the transplant was observed. In some transplants, dense astrocyte accumulation was seen. In all cases (n=52), the response of the recipient’s glia to the transplant was observed, but formation of an extensive glial barrier was noted only in one case. Our findings suggest that the entire range of the results determined by individual peculiarities of the transplant growth and recipient’s response should be thoroughly realized when introducing the methods of neurotransplantation into regenerative medicine.
Similar content being viewed by others
References
Behrstock S, Ebert AD, Klein S, Schmitt M, Moore JM, Svendsen CN. Lesion-induced increase in survival and migration of human neural progenitor cells releasing GDNF. Cell. Transplant. 2008;17(7):753-762.
Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol. Cell. Neurosci. 2003;24(3):623-631.
Blaya MO, Tsoulfas P, Bramlett HM, Dietrich WD. Neural progenitor cell transplantation promotes neuroprotection, enhances hippocampal neurogenesis, and improves cognitive outcomes after traumatic brain injury. Exp. Neurol. 2015;264:67-81.
Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, Kokaia Z. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells. 2012;30(12):2657-2671.
Dunnett SB, Bjorklund A. Basic transplantation methods in rodent brain. Neural Transplantation Methods. Dunnett SB, Boulton AA, Baker GB, eds. Totowa, 2010. P. 133-148.
Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp. Neurol. 2006;198(2):275-284.
Gaillard A, Jaber M. Is the outgrowth of transplant-derived axons guided by host astrocytes and myelin loss? Cell Adh. Migr. 2007;1(4):161-164.
Gaillard A, Nasarre C, Roger M. Early (E12) cortical progenitors can change their fate upon heterotopic transplantation. Eur. J. Neurosci. 2003;17(7):1375-1383.
Gaillard A, Prestoz L, Dumartin B, Cantereau A, Morel F, Roger M, Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat. Neurosci. 2007;10(10):1294-1299.
Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl. Cell Differ. 2009;48:339-351.
Isenmann S, Brandner S, Sure U, Aguzzi A. Telencephalic transplants in mice: characterization of growth and differentiation patterns. Neuropathol. Appl. Neurobiol. 1996;22(2):108-117.
Karbanová J, Mokrý J, Kotingová L. Neural stem cells transplanted into intact brains as neurospheres form solid grafts composed of neurons, astrocytes and oligodendrocyte precursors. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2004;148(2):217-220.
Kunze A, Achilles A, Keiner S, Witte OW, Redecker C. Two distinct populations of doublecortin-positive cells in the perilesional zone of cortical infarcts. BMC Neurosci. 2015;16:20. doi: https://doi.org/10.1186/s12868-015-0160-8.
Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J. Clin. Invest. 2010;120(1):29-40.
Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7(10):e48001. doi: https://doi.org/10.1371/journal.pone.0048001.
Magavi SS, Lois C. Transplanted neurons form both normal and ectopic projections in the adult brain. Dev. Neurobiol. 2008;68(14):1527-1537.
Michelsen KA, Acosta-Verdugo S, Benoit-Marand M, Espuny-Camacho I, Gaspard N, Saha B, Gaillard A, Vanderhaeghen P. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85(5):982-997.
Nishino H, Hida H, Takei N, Kumazaki M, Nakajima K, Baba H. Mesencephalic neural stem (progenitor) cells develop to dopaminergic neurons more strongly in dopamine-depleted striatum than in intact striatum. Exp. Neurol. 2000;164(1):209-214.
Oertel J, Samii M, Walter GF. Fetal allogeneic dopaminergic cell suspension grafts in the ventricular system of the rat: characterization of transplant morphology and graft-host interactions. Acta Neuropathol. 2004;107(5):421-427.
Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407(3):313-319.
Park JK, Joh TH, Ebner FF. Tyrosine hydroxylase is expressed by neocortical neurons after transplantation. Proc. Natl Acad. Sci. USA. 1986;83(19):7495-7498.
Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi A.L, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688-694.
Pluchino S, Zanotti L, Brini E, Ferrari S, Martino G. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Neurosci. Lett. 2009;456(3):101-106.
Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62(11):1895-1904.
Shetty AK, Hattiangady B. Grafted subventricular zone neural stem cells display robust engraftment and similar differentiation properties and form new neurogenic niches in the young and aged hippocampus. Stem Cells Transl. Med. 2016;5(9):1204-1215.
Sukhinich KK, Kosykh AV, Aleksandrova MA. Differentiation and cell-cell interactions of neural progenitor cells transplanted into intact adult brain. Bull. Exp. Biol. Med. 2015;160(1):115-122.
Thompson LH, Björklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol. Dis. 2015;79:28-40.
Unal-Cevik I, Kilinç M, Gürsoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015(1-2):169-174.
Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016;7. ID 10472. doi: https://doi.org/10.1038/ncomms10472.
Wuttke TV, Markopoulos F, Padmanabhan H, Wheeler AP, Murthy VN, Macklis JD. Developmentally primed cortical neurons maintain fidelity of differentiation and establish appropriate functional connectivity after transplantation. Nat. Neurosci. 2018;21(4):517-529.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 3, pp. 164-175, September, 2018
Rights and permissions
About this article
Cite this article
Sukhinich, K.K., Aleksandrova, M.A. Individual Peculiarities of the Development and Differentiation of Embryonic Neocortex Transplants in Intact Adult Mouse Brain. Bull Exp Biol Med 166, 141–150 (2018). https://doi.org/10.1007/s10517-018-4303-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4303-7