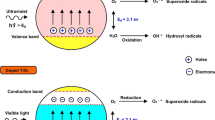
Abstract
Photocatalytic cement is self-cleaning due to the addition of titanium dioxide (TiO2) nanoparticles, which react with sunlight (UV) and produce reactive oxygen species (ROS). Construction workers using photocatalytic cement are exposed not only to cement particles that are irritants but also to nano TiO2 and UV, both carcinogens, as well as the generated ROS. Quantifying ROS generated from added nano TiO2 in photocatalytic cement is necessary to efficiently assess combined health risks. We designed and built an experimental setup to generate, under controlled environmental conditions (i.e., temperature, relative humidity, UV irradiance), both regular and photocatalytic cement aerosols. In addition, cement working activities—namely bag emptying and concrete cutting—were simulated in an exposure chamber while continuously measuring particle size distribution/concentration with a scanning mobility particle sizer (SMPS). ROS production was measured with a newly developed photonic sensing system based on a colorimetric assay. ROS production generated from the photocatalytic cement aerosol exposed to UV (3.3∙10−9 nmol/pt) was significantly higher than for regular cement aerosol, either UV-exposed (0.5∙10−9 nmol/pt) or not (1.1∙10−9 nmol/pt). Quantitatively, the level of photocatalytic activity measured for nano TiO2-containing cement aerosol was in good agreement with the one obtained with only nano TiO2 aerosol at similar experimental conditions of temperature and relative humidity (around 60%). As a consequence, we recommend that exposure reduction strategies, in addition to cement particle exposures, also consider nano TiO2 and in situ–generated ROS, in particular if the work is done in sunny environments.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology—the study of matter in nano range from 1 to 100 nm—is widely used to improve materials’ properties especially strength, weight, and insulation. In the construction sector, photocatalytic cement has been introduced for its self-cleaning properties (Lan et al. 2013; Carp et al. 2004; Banerjee et al. 2015) related to the photocatalytic activity of titanium dioxide nanoparticles (nano TiO2) (Hernández-Rodríguez et al. 2019; Feng et al. 2013; Folli et al. 2010). This cement is composed of regular cement made up of fine inorganic particles such as CaO, SiO2, Fe2O3, and MgO (Meo 2004; Batsungnoen et al. 2019) and nano TiO2.
It is well-known that inhalation of particulate matter (PM) is associated with pulmonary and cardiovascular diseases (e.g., COPD, asthma, lung cancer) (Risom et al. 2005; Aust et al. 2002; Schins et al. 2004; Ghio and Devlin 2001; Knaapen et al. 2004; Upadhyay et al. 2003; Upadhyay et al. 2003; Park et al. 2018). In addition, IARC has PM as a group 1 carcinogen (IARC 2017) and TiO2 as possibly carcinogenic to humans (2B) (IARC 2015). Numerous studies have shown that nano TiO2 is genotoxic and cytotoxic (NIOSH 2009; Sayes et al. 2006), especially for the lung bronchial epithelial cells (Sha et al. 2015; Lee et al. 2010) but can also translocate to other organs via the blood circulation (Wang et al. 2008; Kreyling et al. 2010; Geiser and Kreyling 2010; Shi et al. 2013).
Cell toxicity associated with nano TiO2 exposure is related to reactive oxygen species (ROS) generation, which may lead to oxidative stress, lipid peroxidation, and nucleic acid alteration (Wang and Fan 2014; Shi et al. 2013; Panieri and Santoro 2016; Liou and Storz 2010). ROS such as hydroxyl radical, superoxide anion radical, hydrogen peroxide (H2O2), and singlet oxygen play a mechanistic role in many human diseases, including cancer (Waris and Ahsan 2006; Brieger et al. 2012), especially in the initiation and progression of multistage carcinogenesis (Waris and Ahsan 2006). Elevated ROS levels have also been associated with various inflammation-related human diseases (Alfadda and Sallam 2012).
ROS are also generated outside of the body, and has to be considered together with the endogenous ROS exposure generated through the metabolic response. Environmental ROS generation is especially relevant when airborne nano TiO2 particulates are exposed to UV (Vernez et al. 2017). Due to its electronic energy band gap, nano TiO2 behaves as a semi-conductor: UV-excited electrons (ē) reach the conductance band while a hole (h+) forms at the valence energy level. The resulting ē/h+ pair reacts with molecular oxygen (O2) and water giving rise to a series of ROS formation. They react readily with organic materials (e.g., bacteria and mold), giving them a particularly efficient biocide property (Li et al. 2014; Lan et al. 2013; Li 2004; Chen and Poon 2009; Lee et al. 2010).
Photocatalytic cement exposure among outdoor construction workers may thus have direct exposures to ROS as secondary airborne toxicant (exogenous ROS) from UV activation of nano TiO2. Concentrations of exogenous ROS have not yet been assessed for these workers; consequently, potential health risks associated to this exposure are currently unknown.
Airborne ROS can be quantified using a photonic detection device that was developed at our laboratory and which relies on the formation of a colorimetric complex (Fe (III)-orange xylenol) due to the oxidation of the probe solution containing reduced iron form (Fe (II)) by ROS (Laulagnet et al. 2015). The use of multiscattering absorbance enhancement (MAE) strategy as photonic core principle for the device enabled sensitive ROS determination (Suárez et al. 2013, 2014).
The main objective of the present study was to quantify amount of ROS generated from airborne cement and photocatalytic particles at constant relative humidity of about 60% under controlled conditions:
-
i)
Laboratory aerosolization with photocatalytic and regular cements equipped with a UV lamp;
-
ii)
Exposure chamber setup where two construction activities (cement bag emptying and concrete cutting) were simulated with both cement types separately.
Material and methods
Materials
Photocatalytic cement was obtained from ESSROC (TX-Active®, Italcementi group, Nazareth, US), while regular cement defined as Portland cement CEM I (CE number 266–043-4) was purchased from Jura cement (Wildegg, Switzerland). The ROS-detection reagent—so-called FOX solution—was freshly prepared by mixing ammonium iron (Fe (II)) sulfate (260 μM), xylenol orange (130 μM), and d-sorbitol (100 mM) into sulfuric acid (25 mM). The solution was kept in a fumed glass flask (100 mL). UV exposure was achieved using a solar light simulator (LS-1000 Solar Simulator Solar Light Co., Glenside, PA, USA). Jet-nebulizer system (1-jet Collison Mesa Labs, Butler, NJ; USA) was used to maintain controlled aerosol humidity to 60%. The Ecolog TH1 device enabled monitoring of both temperature and humidity during the aerosol generation (ELPRO-BUCHS AG, Buchs, Switzerland). System airflows were monitored using digital mass flow meters (Vögtlin Instruments AG, flow technology, Aesch BL, Switzerland). Concrete was made by mixing cement and water (2:1). Concrete cutting was operated with a circular saw (diameter 230 mm) and at maximum rated speed (6600 RPM) (PWS 20-230 J, BOSCH, Leinfelden-Echterdingen, Germany).
Methods
Generation of cement aerosols
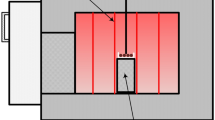
Airborne particles of both photocatalytic and regular cements were generated using an aerosolization system previously described by Ding and Riediker 2015, 2016. Two grams of cement were loaded into a glass funnel and dry air blown upwards through the funnel with 2 L/min. The experimental setup is shown in Fig. 1.
Setup of cement powder experiment. Cement powder gets aerosolized in the glass funnel (2 L/min) by a gentile airstream (Ding and Riediker 2015, 2016). The main air stream was split into one leading to the SMPS for measuring particle number concentration (11–1083 nm), and a second driving the aerosol to the mixing chamber. The particles were mixed with humid air and transported into the UV-exposure cylinder (solar simulator lamp). Temperature and humidity were monitored after the air passed through the UV-cylinder. The airborne particles were captured in an impinger filled with FOX solution and the associated ROS production analyzed with the oxidative potential analyzer system (Laulagnet et al. 2015, Vernez et al. 2017)
Control of environmental conditions
The airborne particles produced in the funnel were transported directly into a mixing chamber by shear force and mixed with humid air originated from a nebulizer. The nebulizer flow rate was 1.5 L/min, which maintained the relative humidity at 60%. Downstream, the aerosol was driven into the exposure cylinder where they were exposed to UV radiation for 2.7 min (average residence time in the cylinder). The UV radiation light source was equipped with solar UV filters to reproduce the UV-A and UV-B spectrum. The lamp produced an irradiance intensity of 785 W/m2 in the cylinder, corresponding to 12 folds the terrestrial irradiance. The airborne particles exiting the cylinder were captured in an impinger (25 mL) filled with FOX solution (5 mL). Temperature and relative humidity were monitored continuously during the run.
Working activities
Two construction activities, cement bag emptying and concrete cutting, were simulated in an exposure chamber (10 m3) with either photocatalytic or regular cement. Prior to the simulation activities, the ventilation system (80 m3/h) was running for 2 h in order to reduce background particles, and during simulation, the ventilation system was off. The operator simulating the construction activity wore a respirator (N100, P3, or FFP3), a chemical suit, nitrile gloves, goggles, safety shoes, and hearing protection. The bag emptying activity was performed by turning an open cement bag (25 kg) upside-down, pouring it into a plastic container (diameter × height, 60 × 40 cm), and shaking until the cement bag was empty. The concrete cutting activity was performed by using a circular saw for 10 s cutting a prepared concrete block (size 25 × 36 × 6 cm). The aerosolized cement particles were sampled in the operator’s breathing zone with an impinger (25 mL) containing FOX solution (5 mL) and operating at a flow rate of 0.5 L/min. Each experimental construction activity was repeated in triplicate by a single operator, as shown in Fig. 2.
Experimental setup to characterize work-generated particle emissions. Two activities, bag emptying and concrete cutting, were reproduced experimentally in the exposure cabin. Workers were wearing whole body protection with personal protective equipment (PPE): dust protection cloth, rubber gloves, goggles, safety shoes, ears muff, and respirator
ROS analysis
ROS concentration—also defined as oxidative potential—was determined using a photonic system developed by our laboratory and based on multiscattering-enhanced absorbance strategy (Laulagnet et al. 2015; Vernez et al. 2017). In brief, air samples are bubbled through an impinger filled FOX solution (5 mL), which is the reaction medium. In the presence of ROS, the Fe (II) undergoes oxidized into Fe (III) that forms a complex with orange xylenol absorbing light at 580 nm. The color change is measured via the use of a narrow emission led (580 nm) coupled to a photodetector both driven through a microcontroller board (Arduino Uno) The multiscattering regime occurring in the photonic cell due to the combination of rough aluminum cavity and inner Teflon housing enables dramatic lengthening of the optical path and improved analytical sensitivity. The ROS sensor response was calibrated with H2O2 and ROS values expressed as H2O2 equivalents.
Particles measurements
The size distribution and number concentration of airborne nanoparticles in the size range between 11 and 1083 nm were measured by a scanning mobility particle sizer (SMPS) (model SMPS+C model 5400, Grimm Aerosol Technik GmbH & Co. KG, Ainring, Germany). For morphology determination, the particles were collected onto Transmission Electron Microscopy (TEM) grids (Quantifoil R1/4, Quantifoil Micro Tools GmbH, Germany) using a mini particle sampler (MPS) (Ecomesure, Sacly, France) operating at a sampling flow rate of 0.3 L/min. The TEM grids were transferred to a transmission electron microscope (TEM) (TEM CM-100 (JEOL, USA) at 80 kV).
Statistical analysis
Means and standard deviations for nanoparticle size and distribution as well as ROS concentrations were compared by two-sample t test using STATA version 15.
Results
The ROS detection system developed by our group enabled us to quantify hydrogen peroxide (H2O2) and hydroxyl radicals (OH•). Quantitative determination of the aerosol reactivity expressed—once normalized by total particle number concentration—in nanomoles of H2O2 equivalents per particle (nmol/pt) was possible by combining accurate aerosol generation and sensitive ROS detection.
The average size distribution from aerosolized photocatalytic cement in the experimental setup was around 2∙105 pt/cm3, with a geometric mean diameter (GMD) of 285 nm and a geometric standard deviation (GSD) of 1.65 nm. Regular cement aerosol had a particle number concentration of 1∙105 pt/cm3 with GMD and GSD of 376 and 1.74 nm, respectively (Fig. 3a). The TEM images confirmed that photocatalytic cement aerosols contained agglomerates from pristine nanoparticles with primary size around 50 nm (Fig. 3b).
(a) Airborne nanoparticle size distribution expressed in number concentration (dN (/cm3)) obtained by SMPS for photocatalytic cement (solid circles) and regular cement (solid triangles) in the range size between 11 and 1083 nm. TEM images of (b) photocatalytic cement and (c) regular cement; (magnification, × 66,000)
In complement, the aerosol ROS generation calculated in the present study indicates that the aerosolized photocatalytic cement exposed to UV irradiance (3.3∙10−9 nmol/pt) is significantly more reactive in terms of produced H2O2 equivalents than regular cement exposed to UV (0.5∙10−9 nmol/pt) or not (1.1∙10−9 nmol/pt). ROS generation for non-UV-exposed cement aerosols were 1.6∙10−9 nmol/pt and 1.1∙10−9 nmol/pt for photocatalytic and regular cement, respectively (Table 1). In good agreement with a prior study (Vernez et al. 2017), the results herein obtained clearly indicate that the presence of nano TiO2 in the photocatalytic cement do increase its chemical reactivity in terms of ROS generation prompt to act as secondary toxicants.
The effect of UV irradiance on nano TiO2 is manifest in the fact that ROS generation from photocatalytic cement doubled in the presence of UV irradiance. As expected, ROS production from photocatalytic cement exposed to UV was significantly higher than regular cement with or without UV (Fig. 4). There was no significant difference in ROS generation between regular cement exposed and not to UV light.
Simulated construction work activities performed in exposure chamber to evaluate airborne ROS levels show that for bag emptying activity, the measured ROS production was significantly greater (p value = 0.04) for photocatalytic (4.6∙10−10 nmol/pt) than for regular cement (1.5∙10−10 nmol/pt) during bag emptying (Fig. 5). In the case of concrete cutting, no significant difference was observed between photocatalytic and regular cement, with ROS reactivities of 1.1∙10−10 and 1.1∙10−10 nmol/pt, respectively (Table 2).
Discussion
Airborne photocatalytic cement particles are a potential source of ROS that is further enhanced in the presence of UV irradiance, and is mainly attributed to the presence of nano TiO2 on the airborne particles (Batsungnoen et al. 2019). The photo-induced mechanism that triggers the production of ROS—mainly in the form of H2O2—at the surface of TiO2 is well-established (Kakinoki et al. 2004, Ghadiry et al. 2016) and has recently been demonstrated for nano TiO2 airborne particles in our prior study (Vernez et al. 2017). The results obtained herein clearly indicate that the presence of nano TiO2 in the photocatalytic cement increase its chemical reactivity in terms of ROS generation, which is in good agreement with this prior study (Vernez et al. 2017).
In the absence of UV irradiance, the ROS generation associated to photocatalytic cement aerosol is not significantly different than the one obtained with regular cement, exposed or not. However, the large interval observed in the ROS production by photocatalytic aerosol without UV exposure might be attributed to the activation of nano TiO2 by visible light during experimental measurements (Etacheri et al. 2015). Consequently, indoor construction workers using photocatalytic cement under artificial light might have greater exposures to ROS compared with workers using regular cement, although to a far lesser extent than outdoor workers.
The low reactivity observed with regular cement particles could potentially be originated from redox reactions in which transition metal in its composition—namely iron oxide (2% Fe2O3)—are prone to take part (Batsungnoen et al. 2019). While many studies have demonstrated the ability of iron oxides particles—such as Fe2O3 and to a greater extent Fe3O4—to activate H2O2 into highly reactive hydroxyl radical via their so-called peroxidase-like behavior (Gao et al. 2017; Pham et al. 2012), to our knowledge, the contribution of iron oxide in the generation of exogenous ROS by Portland cement particles was not yet reported.
In the case of work activities, the ROS production observed during bag emptying with photocatalytic cement was three-fold greater than the one measured with regular cement. Again, even in the absence of UV irradiance, this photocatalytic activity may be attributed to the visible light energy present in the experimental setup, though nano-TiO2 can produce ROS also under dark conditions (Kakinoki et al. 2004). More interestingly, one can notice that in the case of concrete cutting no significant difference is shown between photocatalytic and regular concretes, while in parallel, the corresponding TiO2 contents in the generated aerosols are relatively low (max. 2%) as shown in Table 2. The different TiO2 content observed depending on the work activity is explained by the fact that bag emptying process favors the smaller size fraction to remain airborne (16.5% of TiO2 detected airborne), while the larger cement powder particles will sediment rapidly. In contrast, aerosols created from cement concrete cutting roughly reflects the initial composition of the initial cement powder in the bag (2.0% nano TiO2) because the TiO2 has become part of the cement matrix and is no longer present as individual nano TiO2 particles. It is worthily to notice that the ROS concentration measured during bag emptying using photocatalytic cement was in the same order of magnitude than the value obtained in prior work with pure nano TiO2 once normalized by TiO2 content (Vernez et al. 2017).
Finally, health effects related to airborne nano TiO2 exposure in photocatalytic cement should integrate its reactivity—in the presence of environmental UV/vis irradiance—by considering the associated ROS products as secondary airborne toxicants. In terms of toxic effects, ROS are associated to various metabolic/pathological paths such as oxidative stress, inflammation, genotoxicity, cytotoxicity, DNA damage, and cancer (Li et al. 2014; Brieger et al. 2012; Scherz-Shouval and Elazar 2011; Jaeger et al. 2012; Yin et al. 2012; Jaeger et al. 2012; Wang and Fan 2014).
Conclusion
The combination of an efficient aerosol generation setup coupled with a solar simulation lamp and a sensitive photonic detection device made it possible to assess the production of ROS by photocatalytic and regular cement aerosols. As expected, the presence of nano TiO2 in photocatalytic cement has a strong impact on the ability of the corresponding aerosol to produce exogenous airborne ROS in the presence of UV light. Moreover, the level of ROS generated during work activities was found to be linked to the amount of airborne nano TiO2 present in the cement aerosol. Thus, concrete cutting activities appear to be considerably less problematic in terms of ROS production than bag emptying for which the nano TiO2 content in the aerosol reaches 16%. Considering the photoreactivity of aerosolized photocatalytic cement under UV irradiance and the high content of airborne nano TiO2 generated during bag emptying, worker protection procedures should not only consider nano TiO2 exposure but also its ability to produce ROS as secondary airborne potential toxicants. Providing the specific reactivity of its aerosol under environmental conditions, photocatalytic cement should not only be considered a novel promising material but also a potential new hazard in construction sites.
References
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease [research article]. Biomed Res Int 2012:1–14. https://doi.org/10.1155/2012/936486
Aust AE, Ball JC, Hu AA, Lighty JS, Smith KR, Straccia AM, Veranth JM, Young WC (2002) Particle characteristics responsible for effects on human lung epithelial cells. Res Rep Health Eff Inst 110:1–65 discussion 67-76
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl Catal B Environ 176–177:396–428. https://doi.org/10.1016/j.apcatb.2015.03.058
Batsungnoen K, Hopf NB, Suárez G, Riediker M (2019) Characterization of nanoparticles in aerosolized photocatalytic and regular cement. Aerosol Sci Technol 53(5):540–548. https://doi.org/10.1080/02786826.2019.1578334
Brieger K, Schiavone S, Miller FJ, Krause K-H (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659. https://doi.org/10.4414/smw.2012.13659
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32(1–2):33–177. https://doi.org/10.1016/j.progsolidstchem.2004.08.001
Chen J, Poon C (2009) Photocatalytic construction and building materials: from fundamentals to applications. Build Environ 44(9):1899–1906. https://doi.org/10.1016/j.buildenv.2009.01.002
Ding Y, Riediker M (2015) A system to assess the stability of airborne nanoparticle agglomerates under aerodynamic shear. J Aerosol Sci 88:98–108. https://doi.org/10.1016/j.jaerosci.2015.06.001
Ding Y, Riediker M (2016) A system to create stable nanoparticle aerosols from nanopowders. JoVE 113:e54414–e54414. https://doi.org/10.3791/54414
Etacheri V, Di Valentin C, Schneider J, Bahnemann D, Pillai SC (2015) Visible-light activation of TiO2 photocatalysts: advances in theory and experiments. J Photochem Photobiol C: Photochem Rev 25:1–29. https://doi.org/10.1016/j.jphotochemrev.2015.08.003
Feng D, Xie N, Gong C, Leng Z, Xiao H, Li H, Shi X (2013) Portland cement paste modified by TiO2 nanoparticles: a microstructure perspective. Ind Eng Chem Res 52(33):11575–11582. https://doi.org/10.1021/ie4011595
Folli A, Pochard I, Nonat A, Jakobsen UH, Shepherd AM, Macphee DE (2010) Engineering photocatalytic cements: understanding TiO2 surface chemistry to control and modulate Photocatalytic performances. J Am Ceram Soc 93(10):3360–3369. https://doi.org/10.1111/j.1551-2916.2010.03838.x
Gao L, Fan K, Yan X (2017) Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics 7(13):3207–3227. https://doi.org/10.7150/thno.19738
Geiser M, Kreyling WG (2010) Deposition and biokinetics of inhaled nanoparticles. Particle Fibre Toxicol 7:2. https://doi.org/10.1186/1743-8977-7-2
Ghadiry M, Gholami M, Choon Kong L, Wu Yi C, Ahmad H, Alias Y (2016) Nano-anatase TiO2 for high performance optical humidity sensing on chip. Sensors 16(1):39. https://doi.org/10.3390/s16010039
Ghio AJ, Devlin RB (2001) Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med 164(4):704–708. https://doi.org/10.1164/ajrccm.164.4.2011089
Hernández-Rodríguez MJ, Santana Rodríguez R, Darias R, González Díaz O, Pérez Luzardo JM, Doña Rodríguez JM, Pulido Melián E (2019) Effect of TiO2 addition on mortars: characterization and photoactivity. Appl Sci 9(13):2598. https://doi.org/10.3390/app9132598
IARC (2015) Agents classified by the IARC monographs. World Health Organization, International Agency for Research on Cancer, vol 1–114. http://monographs.iarc.fr/ENG/Classification/
IARC (2017) IARC Monographs on the evaluation of carcinogenic risk to human; list of classification. World Health Organization, International Agencyfor Research on Cancer. vol 1–120. http://monographs.iarc.fr/ENG/Classification/latest_classif.php
Jaeger A, Weiss DG, Jonas L, Kriehuber R (2012) Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 296(1):27–36. https://doi.org/10.1016/j.tox.2012.02.016
Kakinoki K, Yamane K, Teraoka R, Otsuka M, Matsuda Y (2004) Effect of relative humidity on the Photocatalytic activity of titanium dioxide and photostability of famotidine. J Pharm Sci 93(3):582–589. https://doi.org/10.1002/jps.10575
Knaapen AM, Borm PJA, Albrecht C, Schins RPF (2004) Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer 109(6):799–809. https://doi.org/10.1002/ijc.11708
Kreyling WG, Hirn S, Schleh C (2010) Nanoparticles in the lung. Nat Biotechnol 28(12):1275–1276. https://doi.org/10.1038/nbt.1735
Lan Y, Lu Y, Ren Z (2013) Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2(5):1031–1045. https://doi.org/10.1016/j.nanoen.2013.04.002
Laulagnet A, Sauvain JJ, Concha-Lozano N, Riediker M, Suárez G (2015) Sensitive photonic system to measure oxidative potential of airborne nanoparticles and ROS levels in exhaled air. Procedia Eng 120:632–636. https://doi.org/10.1016/j.proeng.2015.08.659
Lee J, Mahendra S, Alvarez PJJ (2010) Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano 4(7):3580–3590. https://doi.org/10.1021/nn100866w
Li G (2004) Properties of high-volume fly ash concrete incorporating nano-SiO2. Cem Concr Res 34(6):1043–1049. https://doi.org/10.1016/j.cemconres.2003.11.013
Li M, Yin J-J, Wamer WG, Lo YM (2014) Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J Food Drug Anal 22(1):76–85. https://doi.org/10.1016/j.jfda.2014.01.006
Liou G-Y, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496. https://doi.org/10.3109/10715761003667554
Meo SA (2004) Health hazards of cement dust. Saudi Med J 25(9):1153–1159
NIOSH (2009) Approaches to safe nanotechnology managing the health and safety concerns associated with engineered nanomaterials. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication 2009:125. http://www.cdc.gov/niosh/docs/2009-125/pdfs/2009-125.pdf
Panieri E, Santoro MM (2016) ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7(6):e2253. https://doi.org/10.1038/cddis.2016.105
Park J, Park EH, Schauer JJ, Yi S-M, Heo J (2018) Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ Int 117:276–283. https://doi.org/10.1016/j.envint.2018.05.018
Pham AL-T, Doyle FM, Sedlak DL (2012) Kinetics and efficiency of H2O2 activation by iron-containing minerals and aquifer materials. Water Res 46(19):6454–6462. https://doi.org/10.1016/j.watres.2012.09.020
Risom L, Møller P, Loft S (2005) Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592(1):119–137. https://doi.org/10.1016/j.mrfmmm.2005.06.012
Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, Warheit DB, Colvin VL (2006) Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci 92(1):174–185. https://doi.org/10.1093/toxsci/kfj197
Scherz-Shouval R, Elazar Z (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36(1):30–38. https://doi.org/10.1016/j.tibs.2010.07.007
Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V (2004) Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol 195(1):1–11. https://doi.org/10.1016/j.taap.2003.10.002
Sha B, Gao W, Cui X, Wang L, Xu F (2015) The potential health challenges of TiO2 nanomaterials. J Appl Toxicol 35:1086–1101. https://doi.org/10.1002/jat.3193
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Particle Fibre Toxicol 10:15. https://doi.org/10.1186/1743-8977-10-15
Suárez G, Santschi C, Plateel G, Martin OJF, Riediker M (2014) Absorbance enhancement in microplate wells for improved-sensitivity biosensors. Biosens Bioelectron 56:198–203. https://doi.org/10.1016/j.bios.2013.12.063
Suárez G, Santschi C, Slaveykova VI, Martin OJF (2013) Sensing the dynamics of oxidative stress using enhanced absorption in protein-loaded random media. Sci Rep 3:3447. https://doi.org/10.1038/srep03447
Upadhyay D, Panduri V, Ghio A, Kamp DW (2003) Particulate matter induces alveolar epithelial cell DNA damage and apoptosis. Am J Respir Cell Mol Biol 29(2):180–187. https://doi.org/10.1165/rcmb.2002-0269OC
Vernez D, Sauvain J-J, Laulagnet A, Otaño AP, Hopf NB, Batsungnoen K, Suárez G (2017) Airborne nano-TiO2 particles: an innate or environmentally-induced toxicity? J Photochem Photobiol A Chem 343:119–125. https://doi.org/10.1016/j.jphotochem.2017.04.022
Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, Li Y, Li B, Ge C, Zhou G, Gao Y, Zhao Y, Chai Z (2008) Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett 183(1–3):72–80. https://doi.org/10.1016/j.toxlet.2008.10.001
Wang J, Fan Y (2014) Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating. Int J Mol Sci 15(12):22258–22278. https://doi.org/10.3390/ijms151222258
Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinogenesis 5:14. https://doi.org/10.1186/1477-3163-5-14
Yin J-J, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP, Zhao B (2012) Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol 263(1):81–88. https://doi.org/10.1016/j.taap.2012.06.001
Acknowledgments
We greatly appreciated the help from Dr. Nicolas Concha Lozano for morphology study; Mr. Timothee Ndarugendamwo for assisting us in the laboratory and during ROS analysis; and Ms. Nicole Charrier, Mr. Benoit Allaz, Mr. Nicolas Sambiagio, Mr. Elvar Oskarsson, Mr. Antoine Milon, and Mr. Yannick Rodari for all practical laboratory support. We thank the Italcementi group for sending us a free sample.
Funding
This study was supported by the Center for Primary Care and Public Health (Unisanté), Department of Occupational and Environmental Health (formerly known as Institute for Work and Health, IST), Switzerland, together with the Royal Thai Government and the Ministry of Science and Technology, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Batsungnoen, K., Riediker, M., Hopf, N.B. et al. Airborne reactive oxygen species (ROS) is associated with nano TiO2 concentrations in aerosolized cement particles during simulated work activities. J Nanopart Res 22, 204 (2020). https://doi.org/10.1007/s11051-020-04913-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-04913-8