Abstract
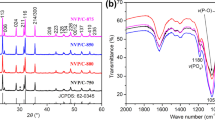
Maricite α-phase sodium cobalt phosphate (α-NaCoPO4) (so called NCP) was fabricated by easily scalable and energy efficient method such as solution combustion synthesis. The obtained material was further ball-milled with carbon (NCP/C) to get nano-sized resultant particles. The orthorhombic crystal structure was confirmed by powder X-ray diffraction and purity of the material evidenced by X-ray photoelectron spectroscopy (XPS). The surface morphology and particle size of the materials were studied by scanning electron and transmission electron microscopic analyses. In cyclic voltammetric analysis, the cathode made up of NCP/C material showed a significant redox activity at 2.33 and 4.3 V. The NCP/C material exhibited a reversible intercalation with a discharge capacity of 36 mAh g−1 at 0.1 C and 50% capacity retention after 100 cycles in Na half-cell. To the best of our knowledge, the maricite α-NaCoPO4 cathode has been taken part in the charge/discharge process with the active sodium ions.

Graphical abstract










Similar content being viewed by others
References
Gutierrez A, Kim S, Fister TT, Johnson CS (2017) Microwave-assisted synthesis of NaCoPO4 red-phase and initial characterization as high voltage cathode for sodium-ion batteries. ACS Appl Mater Interfaces 9(5):4391–4396
Hammond R, Barbier J (1996) Structural chemistry of NaCoPO4. Acta Cryst B 52:440–449
Hui Y, Shewen Y, Yunfeng W, Jiaming Z, Jingwen J, Jiahao C, Qinqin Z, Tongxiang L (2019) Scalable production of hierarchical N-doping porous carbon@cu composite fiber based on rapid gelling strategy for high-performance supercapacitor. J Alloys Compd 792:976–982
Hwang JY, Myung ST, Kook Y (2017) Sodium-ion batteries: present and future. Chem Soc Rev 46:3529–3614
Kim J, Seo DH, Kim H, Park I, Yoo JK, Jung SK, Park YU, Goddard WA III, Kang K (2015) Unexpected discovery of low-cost maricite NaFePO4 as a high-performance electrode for Na-ion batteries. Energy Environ Sci 8:540–545
Le SN, Eng HW, Navrotsky A (2006) Energetics of cobalt phosphate frameworks: α, β, and red NaCoPO4. J Solid State Chem 179:3731–3738
Lee JM, Kim JW, Lim JS, Kim TJ, Kim SD, Park SJ, Lee YS (2007) X-ray photoelectron spectroscopy study of cobalt supported multi-walled carbon nanotubes prepared by different precursors. Carbon Lett 8:120–126
Li W, Zhang B, Lin R, Ho-Kimura SM, He G, Zhou X, Hu J, Parkin IP (2018) A dendritic nickel cobalt sulfide Nanostructurefor alkaline battery electrodes. Adv Funct Mater 28:1705937
Litasov KD, Podgornykh NM (2017) Raman spectroscopy of various phosphate minerals and occurrence of tuite in the Elga IIE iron meteorite. J Raman Spectrosc 48:1518–1527
Liu X, Wang E, Feng G, Wu Z, Xiang W, Guo X, Li J, Zhong B, Zheng Z (2018a) Compared investigation of carbon-decorated Na3V2(PO4)3 with saccharides of different molecular weights as cathode of sodium ion batteries. ElectrochimActa 286:231–241
Liu Y, Zhang N, Wang F, Liu X, Jiao L, Fan LZ (2018b) Approaching the downsizing limit of Maricite NaFePO4 toward high-performance cathode for sodium-ion batteries. Adv Funct Mater 28:1801917
Muruganantham R, Sivakumar M, Subadevi R, Ramaprabhu S, Wu NL (2015) Studies on graphene enfolded olivine composite electrode material via Polyol technique for high rate performance lithium-ion batteries. Electron Mater Lett 11(5):841–852
Ong SP, Chevrier VL, Hautier G, Jain A, Moore C, Kim S, Ma X, Ceder G (2011) Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ Sci 4:3680–3688
Pradeep T (2012) A textbook of Nanoscience and nanotechnology. Tata McGraw Hill Education Private Limited, New Delhi
Rahman MM, Sultana I, Mateti S, Liu J, Sharma N, Chen Y (2017) Maricite NaFePO4/C/graphene: a novel hybrid cathode for sodium-ion batteries. J Mater Chem A 5:16616–16621
Senthilkumar B, Ananya G, Ashok P, Ramaprabhu S (2015) Synthesis of carbon coated nano-Na4Ni3(PO4)2P2O7 as a novel cathode material for hybrid supercapacitors. Electrochim Acta 169:447–455
Sharma L, Bhatia A, Assaud L, Franger S, Barpanda P (2018) Ultra-rapid combustion synthesis of Na2FePO4F fluorophosphate host for Li-ion and Na-ion insertion. Ionics 24:2187–2192
Xu Q, Liu Y, Jiang H, Hu Y, Liu H, Li C (2019) Unsaturated sulfur edge engineering of strongly coupled MoS2 nanosheet–carbon macroporous hybrid catalyst for enhanced hydrogen generation. Adv Energy Mater 9:1802553
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114:11636–11682
Yang H, Ye S, Zhou J, Liang T (2019) Biomass-derived porous carbon materials for supercapacitor. Front Chem 7:274
You Y, Manthiram A (2017) Progress in high-voltage cathode materials for rechargeable sodium-ion batteries. Adv Energy Mater 8:17071785
Acknowledgements
All the authors from Alagappa University were financially supported by DST-SERB, New Delhi, under the Physical Sciences, grant sanctioned vide EMR/2016/006302. All the authors gratefully acknowledge for extending the analytical facilities in the Department of Physics, Alagappa University under the PURSE and FIST programmes, sponsored by Department of Science and Technology (DST) New Delhi, Govt. of India, Alagappa University Research Fund (AURF), Karaikudi, and Ministry of Human Resource Development RUSA- Phase 2.0 grant sanctioned vide Lt.No.F-24-51/2014 U Policy (TNMulti Gen), Dept. of Education, Govt. of India.
Funding
All the authors from Alagappa University were financially supported by DST-SERB, New Delhi, under the Physical Sciences, grant sanctioned vide EMR/2016/006302.
Author information
Authors and Affiliations
Contributions
All the authors equally contributed in terms of framing, planning and executing this research work, including analytical and writing parts.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• To the best of our knowledge, the electrochemical redox activity of maricite α-NaCoPO4 in sodium-ion battery has not yet been reported.
• In this study, we first report the electrochemical performance of maricite α-NaCoPO4 in Na half-cell.
• Upon downsizing the maricite α-NaCoPO4 particle, the sodium ion takes part in cycling.
Electronic supplementary material
ESM 1
(DOCX 8920 kb)
Rights and permissions
About this article
Cite this article
Savithiri, G., Priyanka, V., Subadevi, R. et al. Effect of downsizing the maricite α-phase sodium cobalt phosphate (α-NaCoPO4) in sodium-ion battery. J Nanopart Res 22, 29 (2020). https://doi.org/10.1007/s11051-019-4733-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4733-9