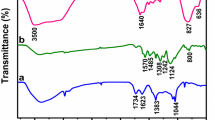
Abstract
There is an intense interest for utilization of self-assembly to fabricate high-density hybrid films for practical energy storage. In this paper, high-density (1.5 mg/cm2) hybrid Na0.44MnO2 nanorod (average diameter 70 nm and average aspect ratio 10)/active carbon films (with area of 9 cm2) were fabricated by direct toluene/water interfacial assembly, achieving the binder-free supercapacitor electrode with advanced electrochemical performance. The density of interfacial films can be precisely controlled by regulating the dosage of assembled nano-units. We showed that the hybrid films can be easily transferred onto a Ni foam and subsequently use as binder-free supercapacitor electrode. The electrochemical testing of film electrode exhibited a high specific capacitance of 189.6 F/g and good capacitance retention at high charge-discharge rates as well as cycling stability. It is suggested that this direct interfacial assembly approach paves the way for the fabrication of 2D functional nanomaterials, particularly useful in practical applications, such as advanced supercapacitor electrode.

Graphical Abstract










Similar content being viewed by others
References
Binks BP (2002) Particles as surfactants-similarities and differences. Curr Opin Colloid Interface Sci 7(1):21–41
Binks BP (2017) Colloidal particles at a range of fluid-fluid interfaces. Langmuir 33(28):6947–6963
Christiansen MUB, Seselj N, Engelbrekt C, Wagner M, Stappen FN, Zhang J (2018) Chemically controlled interfacial nanoparticle assembly into nanoporous gold films for electrochemical applications. J Mater Chem A 6(2):556–564
Dubal DP, Chodankar NR, Kim D-H, Gomez-Romero P (2018) Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem Soc Rev 47(6):2065–2129
Hong X, Zhang B, Murphy E, Zou J, Kim F (2017) Three-dimensional reduced graphene oxide/polyaniline nanocomposite film prepared by diffusion driven layer-by-layer assembly for high-performance supercapacitors. J Power Sources 343:60–66
Hu L, Chen M, Fang X, Wu L (2011) Oil-water interfacial self-assembly: a novel strategy for nanofilm and nanodevice fabrication. Chem Soc Rev 41(3):1350–1362
Jabeen N, Hussain A, Xia Q, Sun S, Zhu J, Xia H (2017) High-performance 2.6 V aqueous asymmetric supercapacitors based on in situ formed Na0.5MnO2 nanosheet assembled nanowall arrays. Adv Mater 29(32):1700804
Lang X, Mo H, Hu X, Tian H (2017) Supercapacitor performance of perovskite La1-xSrxMnO3. Dalton Trans 46(40):13720–13730
Lang X, Zhang H, Xue X, Li C, Sun X, Liu Z, Nan H, Hu X, Tian H (2018) Rational design of La0.85Sr0.15MnO3@NiCo2O4 Core@Shell architecture supported on Ni foam for high performance supercapacitors. J Power Sources 402:213–220
Li Y, Huang W, Sun S (2006) A universal approach for the self-assembly of hydrophilic nanoparticles into ordered monolayer films at a toluene/water interface. Angew Chem Int Ed 45(16):2537–2539
Li Y-J, Liu C, Yang M-H, He Y, Yeung ES (2008) Large-scale self-assembly of hydrophilic gold nanoparticles at oil/water interface and their electro-oxidation for nitric oxide in solution. J Electroanal Chem 622(1):103–108
Li Z, Wang J, Liu X, Liu S, Ou J, Yang S (2011) Electrostatic layer-by-layer self-assembly multilayer films based on graphene and manganese dioxide sheets as novel electrode materials for supercapacitors. J Mater Chem 21(10):3397–3403
Liu C, Li Y-J, Wang M-H, He Y, Yeung ES (2009) Rapid fabrication of large-area nanoparticle monolayer films via water-induced interfacial assembly. Nanotechnology 20(6):065604
Liu C, Li YJ, Sun SG, Yeung ES (2011a) Room-temperature cold-welding of gold nanoparticles for enhancing the electrooxidation of carbon monoxide. Chem Commun 47:4481–4483
Liu S, Fan C-Z, Zhang Y, Li C-H, You X-Z (2011b) Low-temperature synthesis of Na2Mn5O10 for supercapacitor applications. J Power Sources 196(23):10502–10506
Liu C, Li J, Zhao P, Guo W, Yang X (2015) Fast preparation of Na0.44MnO2 nanorods via a high NaOH concentration hydrothermal soft chemical reaction and their lithium storage properties. J Nanopart Res 17:142 10.1007/s11051-015-2954-0
Liu C, Guo W-l, Wang Q-h, Li J-g, Yang X-P (2016) Parametric study of hydrothermal soft chemical synthesis and application of Na0.44MnO2 nanorods for Li-ion battery cathode materials: synthesis conditions and electrochemical performance. J Alloys Compd 658:588–594
Mai L, Li H, Zhao Y, Xu L, Xu X, Luo Y, Zhang Z, Ke W, Niu C, Zhang Q (2013) Fast ionic diffusion-enabled nanoflake electrode by spontaneous electrochemical pre-intercalation for high-performance supercapacitor. Sci Rep 3:1718
Mai L, Tian X, Xu X, Chang L, Xu L (2014) Nanowire electrodes for electrochemical energy storage devices. Chem Rev 114(23):11828–11862
Mao M, Zhou B, Tang X, Chen C, Ge M, Li P, Huang X, Yang L, Liu J (2018) Natural deposition strategy for interfacial, self-assembled, large-scale, densely packed, monolayer film with ligand-exchanged gold nanorods for in situ surface-enhanced Raman scattering drug detection. Chem Eur J 24(16):4094–4102
Milne J, Zhitomirsky I (2018) Application of octanohydroxamic acid for liquid-liquid extraction of manganese oxides and fabrication of supercapacitor electrodes. J Colloid Interface Sci 515:50–57
Mo H, Nan H, Lang X, Liu S, Qiao L, Hu X, Tian H (2018) Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram Int 44(8):9733–9741
Noked M, Liu C, Hu J, Gregorczyk K, Rubloff GW, Lee SB (2016) Electrochemical thin layers in nanostructures for energy storage. Acc Chem Res 49(10):2336–2346
Pang S-C, Anderson MA (2000) Novel electrode materials for electrochemical capacitors: part II. Material characterization of sol-gel-derived and electrodeposited manganese dioxide thin films. J Mater Res 15(10):2096–2106
Park Y-K, Yoo S-H, Park S (2007) Assembly of highly ordered nanoparticle monolayers at a water/hexane interface. Langmuir 23(21):10505–10510
Qu QT, Shi Y, Tian S, Chen YH, Wu YP, Holze R (2009) A new cheap asymmetric aqueous supercapacitor: activated carbon//NaMnO2. J Power Sources 194(2):1222–1225
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Smolin YY, Aken KLV, Boota M, Soroush M, Gogotsi Y, Lau KKS (2017) Engineering ultrathin polyaniline in micro/mesoporous carbon supercapacitor electrodes using oxidative chemical vapor deposition. Adv Mater Interfaces 4(8):1601201
Tuyen N, Michel B, Joao CM, Fatima MM (2017) Layered Ni(OH)2-Co(OH)2 films prepared by electrodeposition as charge storage electrodes for hybrid supercapacitors. Sci Rep 7:39980
Wang S, Liu N, Su J, Li L, Long F, Zou Z, Jiang X, Gao Y (2017a) Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 11(2):2066–2074
Wang Z-H, Yang J-Y, Wu X-W, Chen X-Q, Yu J-G, Wu Y-P (2017b) Enhanced electrochemical performance of porous activated carbon by forming composite with graphene as high-performance supercapacitor electrode material. J Nanopart Res 19:77
Wang Y, Chen H, Wang E (2008) Facile fabrication of gold nanoparticle arrays for efficient surface-enhanced Raman scattering. Nanotechnology 19(10):105604
Whitacre JF, Tevar A, Sharma S (2010) Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem Commun 12(3):463–466
Wu P, Cheng S, Yang L, Lin Z, Gui X, Ou X, Zhou J, Yao M, Wang M, Zhu Y, Liu M (2016) Synthesis and characterization of self-standing and highly flexible δ-MnO2@CNTs/CNTs composite films for direct use of supercapacitor electrodes. ACS Appl Mater Interfaces 8(36):23721–23728
Xia G-G, Tong W, Tolentino EN, Duan N-G, Brock SL, Wang J-Y, Suib SL, Ressler T (2001) Synthesis and characterization of nanofibrous sodium manganese oxide with a 2×4 tunnel structure. Chem Mater 13(5):1585–1592
Xiao F-X, Pagliaro M, Xu Y-J, Liu B (2016) Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: a new perspective for rational construction of multilayer assemblies. Chem Soc Rev 45(11):3088–3121
Xu L, Han G, Hu J, He Y, Pan J, Li Y, Xiang J (2009) Hydrophobic coating- and surface active solvent-mediated self-assembly of charged gold and silver nanoparticles at water-air and water-oil interfaces. Phys Chem Chem Phys 11(30):6490–6497
Zang X, Zhang R, Zhen Z, Lai W, Yang C, Kang F, Zhu H (2017) Flexible, temperature-tolerant supercapacitor based on hybrid carbon film electrodes. Nano Energy 40:224–232
Zou J, Zhang M, Huang J, Bian J, Jie Y, Willander M et al (2018a) Coupled supercapacitor and triboelectric nanogenerator boost biomimetic pressure sensor. Adv Energy Mater 8(10):1702671
Zou Y, Wang Y, Fang Z, Wu D, Yang S, Hao Z, Lang J, Dong Q (2018b) Sulfur powder as a reducing agent to synthesize the Ni@Ni(OH)2 flower-like material for electrochemical capacitors. J Nanosci Nanotechnol 18(11):7732–7738
Acknowledgments
Dr. C Liu thanks Dr. JB Fan in Technical Institute of Physics and Chemistry (CAS) for talking about the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21703013) and Scientific Research Common Program of Beijing Municipal Commission of Education (KM201510017001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1198 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Huang, S., Zhao, K. et al. Ethanol interfacial assembly of Na0.44MnO2 nanorod/active carbon toward the fabrication of high-density hybrid films for binder-free supercapacitor electrode. J Nanopart Res 21, 128 (2019). https://doi.org/10.1007/s11051-019-4571-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4571-9