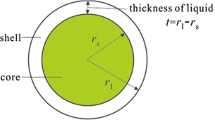
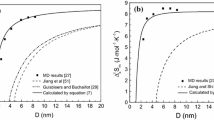
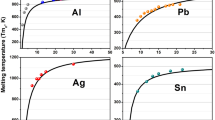
Abstract
A rational melting model is indispensable to address the fundamental issue regarding the melting of nanoparticles. To ascertain the rationality and the application scopes of the three classical thermodynamic models, namely Pawlow, Rie, and Reiss melting models, corresponding accurate equations for size-dependent melting temperature of nanoparticles were derived. Comparison of the melting temperatures of Au, Al, and Sn nanoparticles calculated by the accurate equations with available experimental results demonstrates that both Reiss and Rie melting models are rational and capable of accurately describing the melting behaviors of nanoparticles at different melting stages. The former (surface pre-melting) is applicable to the stage from initial melting to critical thickness of liquid shell, while the latter (solid particles surrounded by a great deal of liquid) from the critical thickness to complete melting. The melting temperatures calculated by the accurate equation based on Reiss melting model are in good agreement with experimental results within the whole size range of calculation compared with those by other theoretical models. In addition, the critical thickness of liquid shell is found to decrease with particle size decreasing and presents a linear variation with particle size. The accurate thermodynamic equations based on Reiss and Rie melting models enable us to quantitatively and conveniently predict and explain the melting behaviors of nanoparticles at all size range in the whole melting process.

Both Reiss and Rie melting models are rational and capable of accurately describing the melting behaviors of nanoparticles at different melting stages. The former is applicable to the stage from initial melting to critical thickness of liquid shell, while the latter from the critical thickness to complete melting. The critical thickness of liquid shell decreases with decreasing particle size and a linear relationship between them is observed. This paper provides us an effective and convenient method to address the fundamental issue regarding the melting temperature of nanoparticles.






Similar content being viewed by others
References
Buffat P, Borel JP (1976) Size effect on the melting temperature of gold particles. Phys Rev A 13(6):2287–2297
Castro T, Reifenberger R, Choi E, Andres RP (1990) Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys Rev B 42(13):8548–8556
Chernyshev AP (2008) Melting of surface layers of nanoparticles: Landau model. Mater Chem Phys 112(1):226–229
Couchman PR, Jesser WA (1977) Thermodynamic theory of size dependence of melting temperature in metals. Nature 269(5628):481–483
Cui ZX, Zhao MZ, Lai WP, Xue YQ (2011) Thermodynamics of size effect on phase transition temperatures of dispersed phases. J Phys Chem C 115(46):22796–22803
Curzon A (1959) PhD Thesis, University of London
Delogu F (2005) Thermodynamics on the nanoscale. J Phys Chem B 109(46):21938–21941
Delogu F (2007) Demixing phenomena in NiAl nanometre-sized particles. Nanotechnology 18(6):065708
Dick K, Dhanasekaran T, Zhang Z, Meisel D (2002) Size-denpendent melting of silica-encapsulated gold nanoparticles. J Am Chem Soc 124(10):2312–2317
Ercolessi F, Andreoni W, Tosatti E (1991) Melting of small gold particles: mechanism and size effects. Phys Rev Lett 66(7):911–914
Font F, Myers TG (2013) Spherically symmetric nanoparticle melting with a variable phase change temperature. J Nanopart Res 15(12):1–13
Goldstein AN, Echer CM, Alivisatos AP (1992) Melting in semiconductor nanocrystals. Science 256(5062):1425–1427
Guenther G, Guillon O (2014) Models of size-dependent nanoparticle melting tested on gold. J Mater Sci 49(23):7915–7932
Guisbiers G, Abudukelimu G (2013) Influence of nanomorphology on the melting and catalytic properties of convex polyhedral nanoparticles. J Nanopart Res 15(2):1431–1442
Guisbiers G, Buchaillot L (2009) Universal size/shape-dependent law for characteristic temperatures. Phys Lett A 374(2):305–308
Guisbiers G, Wautelet M (2006) Size, shape and stress effects on the melting temperature of nano-polyhedral grains on a substrate. Nanotechnology 17(8):192–198
Guisbiers G, Abudukelimu G, Clement F, Wautelet M (2007) Effects of shape on the phase stability of nanoparticles. J Comput Theor Nanosci 4(2):309–315
Hanszen KJ (1960) The melting points of small spherules. Z Phys 157:523–553
Jiang Q, Shi HX, Zhao M (1999) Melting thermodynamics of organic nanocrystals. J Chem Phys 111(5):2176–2180
Jiang Q, Zhang Z, Li JC (2000) Melting thermodynamics of nanocrystals embedded in a matrix. Acta Mater 48(20):4791–4795
Johari GP (1998) Thermodynamic contributions from pre-melting or pre-transformation of finely dispersed crystals. Philos Mag A 77(6):1367–1380
Jones DRH (1974) Free energies of solid-liquid interfaces. J Mater Sci 9(1):1–17
Kuntová Z, Rossi G, Ferrando R (2008) Melting of core-shell Ag-Ni and Ag-Co nanoclusters studied via molecular dynamics simulations. Phys Rev B 77(20):205431
Lai SL, Guo JY, Petrova V, Ramanath G, Allen LH (1996) Size-dependent melting properties of small tin particles: nanocalorimetric measurements. Phys Rev Lett 77(1):99–102
Lai SL, Carlsson JRA, Allen LH (1998) Melting point depression of al clusters generated during the early stages of film growth: nanocalorimetry measurements. Appl Phys Lett 72(9):1098–1100
Lim HS, Ong CK, Ercolessi F (1993) Surface effects in vibrational and melting properties of Pb clusters. Z Phys D 26:45–47
Lindemann FA (1910) The calculation of molecular vibration frequencies. Z Phys 11:609–612
Lubashenko VV (2010) Size-dependent melting of nanocrystals: a self-consistent statistical approach. J Nanopart Res 12(5):1837–1844
Mott NF (1934) The resistance of liquid metals. Proc R Soc Lond A 146(857):465–472
Mottet C, Rossi G, Baletto F, Ferrando R (2005) Single impurity effect on the melting of nanoclusters. Phys Rev Lett 95(3):035501
Nanda KK (2006) A simple classical approach for the melting temperature of inert-gas nanoparticles. Chem Phys Lett 419(1–3):195–200
Nanda KK, Sahu SN, Behera SN (2002) Liquid-drop model for the size-dependent melting of low-dimensional systems. Phys Rev A 66(1):013208
Pavan L, Baletto F, Novakovic R (2015) Multiscale approach for studying melting transitions in CuPt nanoparticles. Phys Chem Chem Phys 17(42):28364–28371
Pawlow P (1909) Relation between melting point and surface energy. Z Phys Chem 65:545–548
Perry RH, Green D (1984) Perry’s chemical engineers’ handbook, 6th. McGraw- Hill, New York, pp 3–128
Reiss H, Wilson IB (1948) The effect of surface on melting point. J Colloid Sci 3:551–561
Rie E (1923) Influence of surface tension on melting and freezing. Z Phys Chem 104:354–362
Rytkonen A, Valkealahti S, Manninen M (1997) Melting and evaporation of argon clusters. J Chem Phys 106(5):1888–1892
Safaei A, Attarian Shandiz M, Sanjabi S, Barber ZH (2007) Modelling the size effect on the melting temperature of nanoparticles, nanowires and nanofilms. J Phys Condens Mat 19(21):216216
Safaei A, Attarian Shandiz M, Sanjabi S, Barber ZH (2008) Modeling the melting temperature of nanoparticles by an analytical approach. J Phys Chem C 112(1):99–105
Sambles JR (1971) An electron microscope study of evaporating gold particles: the Kelvin equation for liquid gold and the lowering of the melting point of solid gold particles. Proc R Soc Lond A 324(1558):339–351
Sinha S, Gao B, Zhou O (2004) Synthesis of silicon nanowires and novel nano-dendrite structures. J Nanopart Res 6(4):421–425
Skripov VP, Koverda VP, Skokov VN (1981) Size effect on melting of small particles. Phys Status Solidi (a) 66(1):109–118
Steenbergen KG, Gaston NA (2016) Two-dimensional liquid structure explains the elevated melting temperatures of gallium nanoclusters. Nano Lett 16(1):21–26
Tang J, Yang J (2015) Simultaneous melting of shell and core atoms, a molecular dynamics study of lithium–copper nanoalloys. J Nanopart Res 17(7):299–310
Wanyika H (2013) Sustained release of fungicide metalaxyl by mesoporous silica nanospheres. J Nanopart Res 15(8):1831–1839
Wautelet M (1998) On the shape dependence of the melting temperature of small particles. Phys Lett A 246(3):341–342
Xiao SF, Hu WY, Yang JY (2006) Melting temperature: from nanocrystalline to amorphous phase. J Chem Phys 125(18):184504/1–184504/4
Xue YQ, Gao BJ, Gao JF (1997) The theory of thermodynamics for chemical reactions in dispersed heterogeneous systems. J Colloid Interface Sci 191(1):81–85
Xue YQ, Zhao QS, Luan CH (2001) The thermodynamic relations between the melting point and the size of crystals. J Colloid Interface Sci 243(2):388–390
Yaws CL (1999) Chemical properties handbook, McGraw-Hill. Book, Beijing, pp (a) 154–157, a78–81, 104–107; b 207–210; c 234–237
Zhang M, Efremov MY, Schiettekatte F, Olson EA, Kwan AT, Lai SL, Wisleder T, Greene JE, Allen LH (2000) Size-dependent melting point depression of nanostructures: nanocalorimetric measurements. Phys Rev B 62(15):10548–10557
Zhang YN, Wang L, Bian XF (2003) Melting of Au nanoclusters by molecular dynamics simulation. Acta Phys -Chim Sin 19(1):35–39
Zhao SJ, Wang SQ, Cheng DY, Ye HQ (2001) Three distinctive melting mechanisms in isolated nanoparticles. J Phys Chem B 105(51):12857–12860
Zhu JH, Fu QS, Xue YQ, Cui ZX (2016) Comparison of different models of melting transformation of nanoparticles. J Mater Sci 51(9):4462–4469
Zhu JH, Fu QS, Xue YQ, Cui ZX (2017) Accurate thermodynamic relations of the melting temperature of nanocrystals with different shapes and pure theoretical calculation. Mater Chem Phys 19:222–228
Acknowledgements
All the authors acknowledge the financial support from the National Natural Science Foundation of China (No. 21373147 and No. 21573157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Table 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Fu, Q., Xue, Y., Cui, Z. et al. Research into the rationality and the application scopes of different melting models of nanoparticles. J Nanopart Res 19, 263 (2017). https://doi.org/10.1007/s11051-017-3960-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3960-1