Abstract
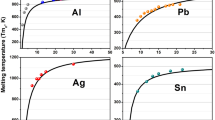
A theoretical model free of any adjustable parameter was derived based on the relation between Gibbs energy change and size to describe the size- and shape-dependent behavior of the melting enthalpy and entropy of nanoparticles. For the melting enthalpy and entropy of vanadium (V), silver (Ag), and copper (Cu) nanoparticles, the results of pure theoretical calculation are in good agreement with available molecular dynamic results. The effect of size on the melting enthalpy and entropy of nanoparticles is greater compared to that of shape effect. The melting enthalpy and entropy decrease with particle size decreasing and the smaller the particle size, the greater the size and shape effects. Furthermore, at the same equivalent diameter, the more the shape of nanoparticles deviates from that of the sphere, the smaller the melting enthalpy and entropy. The thermodynamic relations derived herein can quantitatively describe the influence regularities of size and shape on the melting thermodynamic properties of nanoparticles.




Similar content being viewed by others
References
Couchman PR, Jesser WA (1977) Thermodynamic theory of size dependence of melting temperature in metals. Nature 269:481–483
Guenther G, Guillon O (2014) Models of size-dependent nanoparticle melting tested on gold. J Mater Sci 49:7915–7932. doi:10.1007/s10853-014-8544-1
Shandiz MA, Safaei A, Sanjabi S, Barber ZH (2007) Modeling size dependence of melting temperature of metallic nanoparticles. J Phys Chem Solids 68:1396–1399
Lu HM, Li PY, Cao ZH, Meng XK (2009) Size-, shape-, and dimensionality-dependent melting temperatures of nanocrystals. J Phys Chem C 113:7598–7602
Kaptay G (2012) Nano-Calphad: extension of the Calphad method to systems with nano-phases and complexions. J Mater Sci 47:8320–8335. doi:10.1007/s10853-012-6772-9
Zhu J, Fu Q, Xue Y, Cui Z (2016) Comparison of different models of melting transformation of nanoparticles. J Mater Sci 51:4462–4469. doi:10.1007/s10853-016-9758-1
Lee J, Sim KJ (2013) General equations of CALPHAD-type thermodynamic description for metallic nanoparticle systems. Calphad 44:129–132
Manai G, Delogu F (2007) Homogeneous and heterogeneous melting behavior of bulk and nanometer-sized Cu systems: a numerical study. J Mater Sci 42:6672–6683. doi:10.1007/s10853-007-1522-0
Li X (2014) Modeling the size- and shape-dependent cohesive energy of nanomaterials and its applications in heterogeneous systems. Nanotechnology 25:185702/1–185702/7
Johnston JC, Molinero V (2012) Crystallization, melting, and structure of water nanoparticles at atmospherically relevant temperatures. J Am Chem Soc 134:6650–6659
Pan D, Liu LM, Slater B, Michaelides A, Wang E (2011) Melting the ice: on the relation between melting temperature and size for nanoscale ice crystals. ACS Nano 5:4562–4569
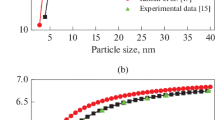
Chernyshev AP (2009) Effect of nanoparticle size on the onset temperature of surface melting. Mater Lett 63:1525–1527
Wang B, Wang G, Chen X, Zhao J (2003) Melting behavior of ultrathin titanium nanowires. Phys Rev B 67:193403/1–193403/4
Buffat P, Borel JP (1976) Size effect on the melting temperature of gold particles. Phys Rev A 13:2287–2298
Lai SL, Guo JY, Petrova V, Ramanath G, Allen LH (1996) Size-dependent melting properties of small tin particles: nanocalorimetric measurements. Phys Rev Lett 77:99–102
Lai SL, Carlsson JRA, Allen LH (1998) Melting point depression of Al clusters generated during the early stages of film growth: nanocalorimetry measurements. Appl Phys Lett 72:1098–1100
Dick K, Dhanasekaran T, Zhang Z, Meisel D (2002) Size-dependent melting of silica-encapsulated gold nanoparticles. J Am Chem Soc 124:2312–2317
Sun PL, Wu SP, Chin TS (2015) Melting point depression of tin nanoparticles embedded in a stable alpha-alumina matrix fabricated by ball milling. Mater Lett 144:142–145
Jiang Q, Shi FG (1998) Entropy for solid–liquid transition in nanocrystals. Mater Lett 37:79–82
Mott NF (1934) The resistance of liquid metals. Proc Royal Soc Lond Ser A 146:465–472
Shi FG (1994) Size dependent thermal vibrations and melting in nanocrystals. J Mater Res 9:1307–1313
Jiang Q, Aya N, Shi FG (1997) Nanotube size-dependent melting of single crystals in carbon nanotubes. Appl Phys A 64:627–629
Kumar R, Sharma G, Kumar M (2013) Effect of size and shape on the vibrational and thermodynamic properties of nanomaterials. J Thermodyn 2013(5):91–99
Safaei A, Shandiz MA (2009) Size-dependent thermal stability and the smallest nanocrystal. Phys E 41:359–364
Xie D, Wang MP, Qi WH, Cao LF (2006) Thermal stability of indium nanocrystals: a theoretical study. Mater Chem Phys 96:418–421
Safaei A, Shandiz MA (2010) Melting entropy of nanocrystals: an approach from statistical physics. Phys Chem Chem Phys 12:15372–15381
Omid H, Hamid DH, Hosseini HRM (2011) Melting enthalpy and entropy of freestanding metallic nanoparticles based on cohesive energy and average coordination number. J Phys Chem C 115:17310–17313
Kofman R, Cheyssac P, Aouaj A, Lereah Y, Deutscher G, Ben-David T, Penisson JM, Bourret A (1994) Surface melting enhanced by curvature effects. Surf Sci 303:231–246
Guisbiers G, Buchaillot L (2009) Modeling the melting enthalpy of nanomaterials. J Phys Chem C 113:3566–3568
Xie D, Qi W, Wang M (2004) Size and shape dependent melting-thermodynamic properties of metallic nanoparticles. Acta Metall Sin 40:1041–1044
Xue YQ, Gao BJ, Gao JF (1997) The theory of thermodynamics for chemical reactions in dispersed heterogeneous systems. J Colloid Interface Sci 191:81–85
Peters KF, Chung YW, Cohen JB (1997) Surface melting on small particles. Appl Phys Lett 71:2391–2393
Mitome M (1999) In-situ observation of melting of fine lead particles by high-resolution electron microscopy. Surf Sci 442:L953–L958
Sheng HW, Lu K, Ma E (1998) Melting and freezing behavior of embedded nanoparticles in ball-milled Al–10wt% M (M = In, Sn, Bi, Cd, Pb) mixtures. Acta Mater 46:5195–5205
Beaglehole D (1991) Surface melting of small particles, and the effects of surface impurities. J Cryst Growth 112:663–669
Son JH, Kim SD, Vij JK, Song JK (2014) Effect of molecular-scale surface morphology on the surface melting of liquid crystals on self-assembled monolayers. Appl Phys Lett 105:251601/1–251601/4
Gülseren O, Ercolessi F, Tosatti E (1995) Premelting of thin wires. Phys Rev B 51:7377–7380
Dash JG (1989) Surface melting. Contemp Phys 30:89–100
Li B, Wang F, Zhou D, Peng Y, Ni R, Han Y (2016) Modes of surface premelting in colloidal crystals composed of attractive particles. Nature 531:485–488
Alarifi HA, Atis M, Ozdogan C, Hu A, Yavuz M, Zhou Y (2013) Determination of complete melting and surface premelting points of silver nanoparticles by molecular dynamics simulation. J Phys Chem C 117:12289–12298
Yaws CL (1999) Chemical Properties Handbook, 1st edn. McGraw-Hill, New York, pp 212–235
Perry RH, Green DW (2008) Perry’s Chemical Engineers’ Handbook, 8th edn. McGraw-Hill, New York, pp 2–136
Tanaka T, Hara S (2001) Thermodynamic evaluation of nano-particle binary alloy phase diagrams. Z Metallkd 92:1236–1241
Yaws CL (1999) Chemical properties handbook. McGraw-Hill, Beijing, pp 154–158
Yaws CL (1999) Chemical properties handbook. McGraw-Hill, Beijing, pp 78–108
Yaws CL (1999) Chemical properties handbook. McGraw-Hill, Beijing, pp 207–211
Yaws CL (1999) Chemical properties handbook. McGraw-Hill, Beijing, pp 234–238
Perry RH, Green D (1984) Perry’s chemical engineers’ handbook, 6th. McGraw-Hill, New York, pp 3–128 Chinese Version
Cui ZX, Zhao MZ, Lai WP, Xue YQ (2011) Thermodynamics of size effect on phase transition temperatures of dispersed phases. J Phys Chem C 115:22796–22803
Tanaka T, Hara S (2001) Thermodynamic evaluation of binary phase diagrams of small particle systems. Z Metallkd 92:467–472
Jiang Q, Yang CC, Li JC (2002) Melting enthalpy depression of nanocrystals. Mater Lett 56:1019–1021
Zhang M, Efremov MY, Schiettekatte F, Olson EA, Kwan AT, Lai SL, Wisleder T, Greene JE, Allen LH (2000) Size-dependent melting point depression of nanostructures: nanocalorimetric measurements. Phys Rev B 62:10548–10557
Luo W, Hu W, Xiao S (2008) Size effect on the thermodynamic properties of silver nanoparticles. J Phys Chem C 112:2359–2369
Delogu F (2005) Structural and energetic properties of unsupported Cu nanoparticles from room temperature to the melting point: molecular dynamics simulations. Phys Rev B 72:205418/1–205418/9
Shandiz MA, Safaei A (2008) Melting entropy and enthalpy of metallic nanoparticles. Mater Lett 62:3954–3956
Acknowledgements
The authors are very grateful for the financial support from the National Natural Science Foundation of China (Nos. 21373147 and 21573157).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fu, Q., Zhu, J., Xue, Y. et al. Size- and shape-dependent melting enthalpy and entropy of nanoparticles. J Mater Sci 52, 1911–1918 (2017). https://doi.org/10.1007/s10853-016-0480-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0480-9