Abstract
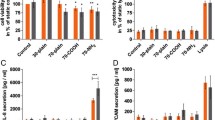
Silica nanoparticles could be promising delivery vehicles for drug targeting or gene therapy. However, few studies have been undertaken to determine the biological behavior effects of silica nanoparticles on primary endothelial cells. Here we investigated uptake, cytotoxicity and angiogenic properties of silica nanoparticle with positive and negative surface charge and sizes ranging from 25 to 115 nm in primary human umbilical vein endothelial cells. Dynamic light scattering measurements and nanoparticle tracking analysis were used to estimate the dispersion status of nanoparticles in cell culture media, which was a key aspect to understand the results of the in vitro cellular uptake experiments. Nanoparticles were taken up by primary endothelial cells in a size-dependent manner according to their degree of agglomeration occurring after transfer in cell culture media. Functionalization of the particle surface with positively charged groups enhanced the in vitro cellular uptake, compared to negatively charged nanoparticles. However, this effect was contrasted by the tendency of particles to form agglomerates, leading to lower internalization efficiency. Silica nanoparticle uptake did not affect cell viability and cell membrane integrity. More interestingly, positively and negatively charged 25 nm nanoparticles did not influence capillary-like tube formation and angiogenic sprouting, compared to controls. Considering the increasing interest in nanomaterials for several biomedical applications, a careful study of nanoparticle-endothelial cells interactions is of high relevance to assess possible risks associated to silica nanoparticle exposure and their possible applications in nanomedicine as safe and effective nanocarriers for vascular transport of therapeutic agents.









Similar content being viewed by others
References
Albini A, Mussi V, Parodi A, Ventura A, Principi E, Tegami S, Rocchia M, Francheschi E, Sogno I, Cammarota R, Finzi G, Sessa F, Noonan DM, Valbusa U (2010) Interactions of single-wall carbon nanotubes with endothelial cells. Nanomed Nanotechnol Biol Med 6(2):277–288. doi:10.1016/j.nano.2009.08.001
Alom-Ruiz SP, Anilkumar N, Shah AM (2008) Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10(6):1089–1100. doi:10.1089/ars.2007.2007
Bagwe RP, Hilliard LR, Tan WH (2006) Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22(9):4357–4362. doi:10.1021/la052797j
Bardi G, Malvindi MA, Gherardini L, Costa M, Pompa PP, Cingolani R, Pizzorusso T (2010) The biocompatibility of amino functionalized CdSe/ZnS quantum-dot-Doped SiO2 nanoparticles with primary neural cells and their gene carrying performance. Biomaterials 31(25):6555–6566. doi:10.1016/j.biomaterials.2010.04.063
Bauer AT, Strozyk EA, Gorzelanny C, Westerhausen C, Desch A, Schneider MF, Schneider SW (2011) Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials 32(33):8385–8393. doi:10.1016/j.biomaterials.2011.07.078
Berry JP, Arnoux B, Stanislas G, Galle P, Chretien J (1977) A microanalytic study of particles transport across the alveoli: role of blood platelets. Biomedicine 27(9–10):354–357
Borselli C, Oliviero O, Battista S, Ambrosio L, Netti PA (2007) Induction of directional sprouting angiogenesis by matrix gradients. J Biomed Mater Res Part A 80A(2):297–305. doi:10.1002/jbm.a.30896
Filipe V, Hawe A, Jiskoot W (2010) Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res 27(5):796–810. doi:10.1007/s11095-010-0073-2
Graf C, Gao Q, Schuetz I, Noufele CN, Ruan W, Posselt U, Korotianskiy E, Nordmeyer D, Rancan F, Hadam S, Vogt A, Lademann J, Haucke V, Ruehl E (2012) Surface functionalization of silica nanoparticles supports colloidal stability in physiological media and facilitates internalization in cells. Langmuir 28(20):7598–7613. doi:10.1021/la204913t
Iijima M, Tsukada M, Kamiya H (2007) Effect of particle size on surface modification of silica nanoparticles by using silane coupling agents and their dispersion stability in methylethylketone. J Colloid Interface Sci 307(2):418–424. doi:10.1016/j.jcis.2006.11.044
Jo DH, Kim JH, Yu YS, Lee TG, Kim JH (2012) Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed Nanotechnol Biol Med 8(5):784–791. doi:10.1016/j.nano.2011.09.003
Kim JA, Åberg C, de Cárcer G, Malumbres M, Salvati A, Dawson KA (2013) Correction to low dose of amino-modified nanoparticles induces cell cycle arrest. ACS Nano. doi:10.1021/nn405243f
Lesniak A, Fenaroli F, Monopoli MR, Aberg C, Dawson KA, Salvati A (2012) Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 6(7):5845–5857. doi:10.1021/nn300223w
Liu X, Sun J (2010) Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kappa B pathways. Biomaterials 31(32):8198–8209. doi:10.1016/j.biomaterials.2010.07.069
Liu T, Liu H, Fu C, Li L, Chen D, Zhang Y, Tang F (2013) Silica nanorattle with enhanced protein loading: a potential vaccine adjuvant. J Colloid Interface Sci 400:168–174. doi:10.1016/j.jcis.2013.03.005
Lu J, Liong M, Zink JI, Tamanoi F (2007) Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 3(8):1341–1346. doi:10.1002/smll.200700005
Lundqvist M, Stigler J, Cedervall T, Berggard T, Flanagan MB, Lynch I, Elia G, Dawson K (2011) The evolution of the protein corona around nanoparticles: a test study. ACS Nano 5(9):7503–7509. doi:10.1021/nn202458g
Luo D, Han E, Belcheva N, Saltzman WM (2004) A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J Controlled Release 95(2):333–341. doi:10.1016/j.jconrel.2003.11.019
Maiorano G, Sabella S, Sorce B, Brunetti V, Malvindi MA, Cingolani R, Pompa PP (2010) Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 4(12):7481–7491. doi:10.1021/nn101557e
Malvindi MA, Brunetti V, Vecchio G, Galeone A, Cingolani R, Pompa PP (2012) SiO2 nanoparticles biocompatibility and their potential for gene delivery and silencing. Nanoscale 4(2):486–495. doi:10.1039/c1nr11269d
Merget R, Bauer T, Kupper HU, Philippou S, Bauer HD, Breitstadt R, Bruening T (2002) Health hazards due to the inhalation of amorphous silica. Arch Toxicol 75(11–12):625–634. doi:10.1007/s002040100266
Monopoli MP, Aberg C, Salvati A, Dawson KA (2012) Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7(12):779–786. doi:10.1038/nnano.2012.207
Montes-Burgos I, Walczyk D, Hole P, Smith J, Lynch I, Dawson K (2010) Characterisation of nanoparticle size and state prior to nanotoxicological studies. J Nanopart Res 12(1):47–53. doi:10.1007/s11051-009-9774-z
Nel AE, Maedler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8(7):543–557. doi:10.1038/nmat2442
Nemmar A, Hoet PHM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B (2002) Passage of inhaled particles into the blood circulation in humans. Circulation 105(4):411–414. doi:10.1161/hc0402.104118
Ponce ML (2009) Tube formation: an in vitro matrigel angiogenesis assay. Methods Mol Biol 467:183–188
Rancan F, Gao Q, Graf C, Troppens S, Hadam S, Hackbarth S, Kembuan C, Blume-Peytavi U, Ruehl E, Lademann J, Vogt A (2012) Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano 6(8):6829–6842. doi:10.1021/nn301622h
Shapero K, Fenaroli F, Lynch I, Cottell DC, Salvati A, Dawson KA (2011) Time and space resolved uptake study of silica nanoparticles by human cells. Mol BioSyst 7(2):371–378. doi:10.1039/c0mb00109k
Silvestri B, Guarnieri D, Luciani G, Costantini A, Netti PA, Branda F (2012) Fluorescent (rhodamine), folate decorated and doxorubicin charged, PEGylated nanoparticles synthesis. J Mater Sci 23(7):1697–1704. doi:10.1007/s10856-012-4634-2
Tan WH, Wang KM, He XX, Zhao XJ, Drake T, Wang L, Bagwe RP (2004) Bionanotechnology based on silica nanoparticles. Med Res Rev 24(5):621–638. doi:10.1002/med.20003
Wang F, Bexiga MG, Anguissola S, Boya P, Simpson JC, Salvati A, Dawson KA (2013a) Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale 5(22):10868–10876. doi:10.1039/c3nr03249c
Wang F, Yu L, Monopoli MP, Sandin P, Mahon E, Salvati A, Dawson KA (2013b) The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomed Nanotechnol Biol Med 9(8):1159–1168. doi:10.1016/j.nano.2013.04.010
Acknowledgments
The authors thank Dr. Olimpia Oliviero for her helpful suggestions in HUVECs cell culture and angiogenesis assays.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniela Guarnieri and Maria Ada Malvindi have equally contributed to this work
Rights and permissions
About this article
Cite this article
Guarnieri, D., Malvindi, M.A., Belli, V. et al. Effect of silica nanoparticles with variable size and surface functionalization on human endothelial cell viability and angiogenic activity. J Nanopart Res 16, 2229 (2014). https://doi.org/10.1007/s11051-013-2229-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2229-6