Abstract
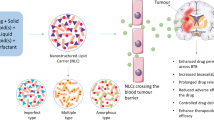
The purpose of this study is to investigate the targeting potential of amino acid (phenylalanine)-coupled solid lipid nanoparticles (SLN) loaded with ionically complexed doxorubicin HCl (Dox). Ionic complexation was used to enhance the loading efficiency and release characteristics of water soluble form of Dox. l-Type amino acid transporters (LAT1) are highly expressed on blood brain barrier as well as on many brain cancer cells, thus targeting LAT1 using phenylalanine improved anticancer activity of prepared nanocarrier. The phenylalanine-coupled SLN were characterized by fourier transform infrared spectroscopy, scanning electron microscope, transmission electron microscopy, particle size, zeta potential, entrapment efficiency and in vitro release. The particle size of the resulting SLN was found to be in the range of 163.3 ± 5.2 to 113.0 ± 2.6 nm, with a slightly negative surface charge. In ex vivo study on C6 glioma cell lines, the cellular cytotoxicity of the SLN was highly increased when coupled with phenylalanine. In addition, stealthing sheath of PEG present on the surface of the SLN enhanced the cellular uptake of the SLN on C6 glioma cell line. Results of biodistribution and fluorescence studies clearly revealed that phenylalanine-coupled SLN could deliver high amount of drug into the brain tumor cells and showed the brain-targeting potential.











Similar content being viewed by others
References
Asano S, Kameyama M, Oura A, Morisato A, Sakai H, Tabuchi Y, Chairoungdua A, Endou H, Kanai Y (2007) l-type amino acid transporter-1 expressed in human astrocytomas, U343MGa. Biolog Pharm Bull 30:415–422
Be′duneaua A, Saulniera P, Benoita JP (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967
Behin A, H-X K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361(9354):323–331
Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM (1999) Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc Natl Acad Sci U 21:79–84
Duelli R, Enerson BE, Gerhart DZ, Drewes LR (2000) Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab 11:1557–1562
Duneaua AB, Saulniera P, Benoit JP (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967
Freshney RI (1983) Culture of animal cells: a manual of basic technique. Alan R Liss, New York, p 112
Fundar`o A, Cavallib R, Bargonic A, Vighettob D, Zaraa GP, Gascob MR (2000) Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after I V administration to rats. Pharmacol Res 42:338–342
Gupta Y, Jain A, Jain SK (2007) Transeferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrocholride to the brain. J Pharm Pharmacology 59:1–6
Jain A, Agarwal A, Majumder S, Lariya N, Khaya A, Agrawal H, Majumdar S, Agrawal GP (2010) Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Controlled Release 148(3):359–367
Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N, Shimohama S (2000) The 4F2hc/LAT1 complex transports l-DOPA across the blood–brain barrier. Brain Res 879:115–121
Kaur IP, Bhandari R, Bhandari S, Kakkar V (2008) Potential of solid lipid nanoparticles in brain targeting. J Control Rel 127:97–109
Lin R, Ng LS, Wang CH (2005) In vitro study of anticancer drug doxorubicin in PLGA-based microparticles. Biomaterials 26:4476–4485
Ling V (1992) p-glycoprotein and resistance to anticancer drugs. Cancer 69:2603–2609
Miglietta A, Cavalli R, Bocca C, Gabriel L, Gasco MR (2000) Cellular uptake and cytotoxicity of solid lipid nanospheres (SLN) incorporating doxorubicin or paclitaxel. Int J Pharm 210:61–67
Misra A, Ganesh S (2003) Drug delivery to the central nervous system: a review. J Pharm Pharm Sci 6:252–273
Moore A, Basilion JP, Chiocca EA, Weissleder R (1998) Measuring transferring receptor gene expression by NMR imaging. Biochim Biophys Acta 1402:239–249
Rautio J, Laine K, (2008) Gynther M Enhanced brain drug delivery and targeting. Pharma Tech Europe
Reddy LH, Murthy RSR (2004) Pharmacokinetics and biodistribution studies of doxorubicin loaded poly (butyl cyanoacrylate) nanoparticles synthesized by two different techniques. Biomed Pap 148:161–166
Reddy LH, Meda N, Murthy RSR (2005) Rapid and sensitive HPLC method for the estimation of doxorubicin in dog blood—the silver nitrate artifac. Acta Pharm 55:81–91
Schoepf U, Marecos E, Melder R, Jain R, Weissleder R (1998) Intracellular magnetic labeling of lymphocytes for in vivo trafficking studies. Biotechniques 24:642
Schubert MA, Goymann CCM (2003) Solvent injection as a new approach for manufacturing lipid nanoparticles evaluation of the method and process parameters. Eur J Pharm Biopharm 55:125–131
Siekmann B, Westesen K (1994) Melt-homogenized solid lipid nanoparticles stabilized by the nonionic surfactant tyloxapol I Preparation and particle size determination. Pharm Pharmacol Lett 3:194–197
Storm G, Belliot SO, Daemen T, Lasic DD (1995) Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev 17:31–48
Weissleder R, Cheng HC, Bogdanova A, Bogdanova A Jr (1997) Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging 7:258–263
Wong HL, Bendayan R, Rauth AM, Xiao YW (2004) Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharm Sci 93(8):1993–2008
Acknowledgments
Authors are thankful to M/s Khandelwal laboratory, Mumbai, India for generously supplying Dox HCl, Indian Institute of Technology, Kanpur for carrying out SEM study. We also wants to acknowledge University Grant Comission, New Delhi for financial assistance to one author (PK).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kharya, P., Jain, A., Gulbake, A. et al. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J Nanopart Res 15, 2022 (2013). https://doi.org/10.1007/s11051-013-2022-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2022-6