Abstract
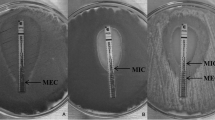
Reference methods for antifungal susceptibility tests recommend the use of conidia as inoculum. However, some isolates produce few conidia, while the invasive form of filamentous fungi in general is hyphae making susceptibility tests infeaseble. These facts suggest that other than conidia broth dilution method is required for susceptibility tests. The aim of this study was to clarify if the hyphal growth inhibition rate could be used as a method of determining the antifungal susceptibility of genus Microsporum. For this reason, a method which traces hyphal tips automatically and measures their growth rate was standardized for Microsporum spp. Control growth curves and test growth curves obtained by real-time observation of the hyphae groups responses to different concentrations of terbinafine, griseofulvin, and ciclopiroxolamine were used to compare with minimum inhibitory concentrations (MICs) obtained by conidia broth microdilution method. A visible reduction in the growth inhibition rate was observed when hyphal activity was evaluated using the third or fourth serial two-fold dilution below the MIC determined by broth microdilution for terbinafine and ciclopiroxolamine. For griseofulvin, this reduction occurred after the fifth dilution below the MIC. This study highlights the importance of the inoculum type used to determine the in vitro susceptibility of Microsporum strains. We conclude that measurement of hyphal growth inhibition, despite being time consuming, could be a suitable method for evaluating antifungal susceptibility, particularly for fungi as Microsporum spp. that produce a small (or not at all) number of conidia.


Similar content being viewed by others
References
Krakhecke AG, Afonso E, Ferreira JC, Candido RC. In vitro susceptibility testing of M gypseum isolates from healthy cattle and soil samples against itraconazole, terbinafine, fluoconazole and topical veterinarian drugs. Mycopathologia. 2005;159:377–80.
Perea S, Fothergill AW, Sutton DA, Rinaldi MG. Comparison of in vitro activities of voriconazole and five established antifungal agents against different species of dermatophytes using a broth macrodilution method. J Clin Microbiol. 2001;39:385–8.
Carrillo-Munoz AJ, Giusiano G, Cárdenes D, et al. Terbinafine susceptibility patterns for onychomycosis-causative dermatophytes and Scopulariopsis brevicaulis. Int J Antimicrob Agents. 2008;31:540–3.
Fernández-Torres B, Carrillo AJ, Martín E, Del Palacio A, Moore MK, Valverde A, Serrano M, Guarro J. In vitro activities of 10 antifungal drugs against 508 dermatophyte strains. Antimicrob Agents Chemother. 2001;45:2524–8.
Fernández-Torres B, Cabanes FJ, Carrillo-Munoz AJ, Esteban A, Inza I, Abarca L, Guarro J. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J Clin Microbiol. 2002;40:3999–4003.
Alió AB, Mendonza M, Zambrano EA, Díaz E, Cavallera E. Dermatophytes growth curve and in vitro susceptibility test: a broth micro-titration method. Med Mycol. 2005;43:319–25.
Araujo CR, Miranda KC, Fernandes OF, Soares AJ, Silva MR. In vitro susceptibility testing of dermatophytes isolates in Goiania, Brazil, against five antifungal agents by broth microdilution method. Rev Inst Med Trop S. Paulo. 2009;51(1):9–12.
Biancalana FS, Telles PF, Lyra L, Schreiber AZ. Preanalytical conditions for broth microdilution antifungal susceptibility of Microsporum spp. Mycoses. 2008;51:313–7.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. CLSI 2008. Approved standard M38-A2.
Teixeira ABA, Moretti ML, Trabasso P, et al. Evaluation of Fusarium solani hyphae and conidia susceptibility to amphotericin B and itraconazole: study of a clinical case. Mycophatologia. 2005;116(4):291–6.
Yamada S, Cao J, Sumita O, et al. Automatic antifungal activity analyzing system on the basis of dynamic growth process of a single hypha. Mycopathologia. 1992;118:65–9.
Wetter TJ, Hazen KC, Cutler JE. Comparison between Aspergillus fumigatus conidia, hyphae susceptibilities to amphotericin B, itraconazole and voriconazole by use of the mold rapid susceptibility assay. Med Mycol. 2005;43:525–32.
Taguchi H, Miyaji M, Nishimura K, Xu M. Studies on the synergistic effect of amphotericin B and 5-fluorocytosine on the growth rate of single hyphae of Aspergillus fumigatus by a Biocell-Tracer® system. Mycoscience. 1995;36:341–4.
Oh K, Yang HC, Matsuoka A, Kurata H. Combined effect of amphotericin B and flucytosine on hypha growth of Candida albicans estimated at a single hypha level. J Med Vet Mycol. 1995;33:1–4.
Oh K, Matsuoka H, Nemoto Y, Sumita O, Takatori K, Kurata H. Determination of anti-Aspergillus activity of antifungal agents based on the dynamic growth rate of a single hypha. Appl Microbiol Biotechnol. 1993;39:363–7.
Teixeira ABA, Moretti ML, Machado HC, Nishimura K, Taguchi H, Schreiber AZ. Evaluation of the inhibitory effect of amphotericin B on the apical growth of F.solani using the BioCell-Tracer® system. Mycoses. 2007;50:183–8.
Iida Y, Oh KB, Saito M, et al. Detection of antifungal activity in Anemarrhena asphodeloides by sensitive BCT method and isolation of its active compound. J Agric Food Chem. 1999;47:584–7.
Bezjak V. Standardization of a hyphal inoculum of Aspergillus for amphotericin B susceptibility testing. J Clin Microbiol. 1985;21(4):509–12.
Ansheng L, Taguchi H, Miyaji M, Nishimura K, Wu S. Study on the hyphal responses of Aspergillus fumigatus to the antifungal agent by Biocell-Tracer®. Mycopathologia. 1999;148:17–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biancalana, F.S.C., Lyra, L., Moretti, M.L. et al. Standardization of Hyphal Growth Inhibition Rate as a Means of Evaluating Microsporum spp. in vitro Susceptibility to Terbinafine, Griseofulvin, and Ciclopiroxolamine. Mycopathologia 172, 279–285 (2011). https://doi.org/10.1007/s11046-011-9433-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-011-9433-7