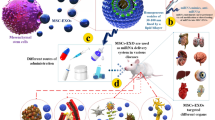
Abstract
Background
RNA (ribonucleic acid) antisense is developing as a possible treatment option. As an RNA, miR-34a is involved in P53 function and cancer cell apoptosis. Although the therapeutic applications of miRNAs have several limitations, such as structural instability and susceptibility to nucleases. To resolve these issues, this study aims to apply exosomes as a delivery vehicle for miR-34a.
Aims
This study aims to create a cell factory to generate miR34a-enriched exosomes. The produced nanoparticles act as a delivery system and improve the structural stability of miR34a.
Methods
First exosome specific sequences were inserted into miR34a. The resulting miR34a oligonucleotide was transduced HEK293T cells genome with a lentiviral system. In the structure of miR34a oligonucleotide, six nucleotides were substituted to increase its packaging rate into exosomes. To maintain the secondary structure, stability, and expression of the miRNA gene, changes to the miR34a oligonucleotide were made using PCR (polymerase chain reaction) Extension. The forward-34a (5-TGGGGAGAGGCAGGACAGG-3) and Reverse-34a primers (5-TCCGAAGTCCTGGCGTCTCC-3) were used for amplification of the miR34a gene from DNA.
Results
The results confirmed that the changes in miR34a oligonucleotide do not affect its secondary structure. The energy level of the manipulated miR34a oligonucleotide was kept the same compared to the original one. Moreover, the loading of miR34a to the exosomes was increased.
Conclusion
Our findings revealed that normal HEK293T did not express miR34a. However, lentiviral transduced miR34a oligonucleotide induced the loading of miR34a into the exosome. Moreover, replacing six nucleic acids in the 3’ end of miR34a increased the loading of miR34a to exosome.






Similar content being viewed by others
Data Availability
All the data are reported as they were obtained. And the original data will be presented upon a reasonable request. The RNA folding (Fig. 1) was obtained from http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi website.
References
Huppi K, Martin SE, Caplen NJ (2005) Defining and assaying RNAi in mammalian cells. Mol Cell 17(1):1–10
Chen F, Hu SJ (2012) Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol 26(2):79–86
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis Proceedings of the National Academy of Sciences, 105(36): p. 13421–13426
Akao Y et al (2011) Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer Lett 300(2):197–204
Fan YN et al (2014) Mir-34a mimics are potential therapeutic agents for p53-mutated and chemo-resistant brain tumour cells. PLoS ONE 9(9):e108514
O’Neill CP, Dwyer RM (2020) Nanoparticle-based delivery of tumor suppressor microRNA for cancer therapy. Cells 9(2):521
Reshke R et al (2020) Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat biomedical Eng 4(1):52–68
Tian Z et al (2021) Insight into the prospects for RNAi therapy of Cancer. Front Pharmacol, 12(308)
Suh JH et al (2021) Therapeutic application of exosomes in inflammatory diseases. Int J Mol Sci 22(3):1144
Munagala R et al (2021) Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett 505:58–72
Sinha D et al (2021) Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers 13(2):326
Wahlgren J et al (2012) Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 40(17):e130–e130
Lotvall J, Valadi H (2007) Cell to cell signalling via exosomes through esRNA. Cell Adhes Migr 1(3):156–158
Lässer C, Eldh M, Lötvall J (2013) The role of exosomal shuttle RNA (esRNA) in cell-to-cell communication. Emerg Concepts Tumor Exosome–Mediated Cell-Cell Communication, : p. 33–45
Villarroya-Beltri C et al (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4(1):2980
Coughlan C et al (2020) Exosome isolation by Ultracentrifugation and Precipitation and techniques for downstream analyses. Curr Protoc Cell Biol 88(1):e110
Limoni SK et al (2019) Engineered Exosomes for targeted transfer of siRNA to HER2 positive breast Cancer cells. Appl Biochem Biotechnol 187(1):352–364
O’Brien J et al (2018) Overview of MicroRNA Biogenesis, Mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9:402
Fu Z et al (2021) MicroRNA as an important target for anticancer drug development. Front Pharmacol, : p. 2212
Holjencin C, Jakymiw A (2022) MicroRNAs and their big therapeutic impacts: delivery strategies for Cancer intervention. Cells 11(15):2332
Sharma P et al (2020) Nanomaterials for autophagy-related miRNA-34a delivery in cancer treatment. Front Pharmacol 11:1141
Li F et al (2018) miR-221 suppression through nanoparticle-based miRNA delivery system for hepatocellular carcinoma therapy and its diagnosis as a potential biomarker. Int J Nanomed 13:2295
Chaudhary V, Jangra S, Yadav NR (2018) Nanotechnology based approaches for detection and delivery of microRNA in healthcare and crop protection. J Nanobiotechnol 16(1):1–18
Fu S et al (2020) Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact 20:100261
Kalfert D et al (2020) Multifunctional roles of miR-34a in cancer: a review with the emphasis on head and neck squamous cell carcinoma and thyroid cancer with clinical implications. Diagnostics 10(8):563
Misso G et al (2014) Mir-34: a new weapon against cancer? Mol therapy-nucleic acids 3:e195
Hermeking H (2010) The miR-34 family in cancer and apoptosis. Cell Death & Differentiation 17(2):193–199
Li M 34a: potent tumor suppressor, cancer stem cell inhibitor, and potential anticancer therapeutic, front. Cell Dev Biol, (9): p. 322
Hong DS et al (2020) Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 122(11):1630–1637
Song B-W, Oh S, Chang W (2022) Multiplexed targeting of microRNA in stem cell-derived extracellular vesicles for regenerative medicine. BMB Rep 55(2):65
Alvarez-Erviti L et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
Wang J-H et al (2018) Anti-HER2 scfv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol Cancer Ther 17(5):1133–1142
Amiri A et al (2022) Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J Translational Med 20(1):1–16
Lamichhane TN et al (2016) Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 9(3):315–324
Raghav A, Jeong G-B (2021) A systematic review on the modifications of extracellular vesicles: a revolutionized tool of nano-biotechnology. J Nanobiotechnol 19(1):1–19
Munir J, Yoon JK, Ryu S (2020) Therapeutic miRNA-enriched extracellular vesicles: current approaches and future prospects. Cells 9(10):2271
Zhang J et al (2015) Exosome and Exosomal MicroRNA: trafficking, sorting, and function. Proteom Bioinf 13(1):17–24Genomics
Bolukbasi MF et al (2012) miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol Therapy-Nucleic Acids 1:e10
Koppers-Lalic D et al (2014) Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep 8:1649–1658
Chen L et al (2020) Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 10(20):9425
Dufait I et al (2012) Retroviral and lentiviral vectors for the induction of immunological tolerance Scientifica, 2012
Acknowledgements
This study was supported financially by Grant No. #2837 from Mazandaran University of Medical Sciences, Sari, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal experiments were approved by the Research and Ethical Committee of Mazandaran University of Medical Sciences, Sari, Iran.
Disclosure of potential conflicts of interest
The authors declare no competing interest.
Research involving human participants and/or animals
This research does not include human tissue or animals.
Informed consent
All the authors consent to publish this article’s findings.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkami, S.A., Molavipordanjani, S., Abediankenari, S. et al. Engineering HEK293T cell line by lentivirus to produce miR34a-loaded exosomes. Mol Biol Rep 50, 8827–8837 (2023). https://doi.org/10.1007/s11033-023-08754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08754-1