Abstract
Esophageal carcinoma (EC) is always diagnosed at advanced stage and its the mortality rate remains high. The patients usually miss the best opportunity for treatment because of non-specific symptoms and the survival rates are low. N6-methyladenosine (m6A) the predominant modification in eukaryotic messenger RNA(mRNA), serves vital roles in numerous bioprocess. This chemical modification is dynamic, reversible and consists of three regulators: m6A methyltransferases (writers), demethylases (erasers) and m6A-binding proteins (readers). Recently, a growing number of evidences have indicated relationships between m6A and EC. Whereas, lacking of cognition about the molecular mechanism of m6A modification in esophageal carcinoma. We will focus on the biological function roles of m6A modification in the tumorigenesis and development of EC. Recent studies showed that immunotherapy had a positive impact on EC. The relationship between m6A and immunotherapy in EC deserves further research and discussion. We will also discuss the potential clinical applications regarding diagnosis, treatment and prognosis of m6A modification for EC and provide perspectives for further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal carcinoma (EC) is one of the most frequent and lethal malignant tumors worldwide. According to the Global Cancer Statistics 2020, approximately 604,000 new cases and 544,000 deaths with the EC patient have been reported each year [1]. The major histological types of EC include esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). The consumption of both tobacco and alcohol greatly could increase the risk of the ESCC in a synergism. Some suspected pathogenic factors for the ESCC include pickled vegetables and hot food [2]. The major proportion of esophageal cancer in high-income countries is EAC and the risk factors of the EAC are gastroesophageal reflux disease, Barrett’s esophagus, obesity and smoking etc. [3]. Early diagnosis of the EC becomes possible by using esophagus endoscopy. However, non-specific symptoms of the EC result in the negligence of the EC during early stage because esophagus endoscopy is not a regular item for annual physical check-ups. Hence the best diagnostic opportunity of the EC is often delayed. The onset of dysphagia is associated with the advanced stage of the EC and its 5-years survival is lower than 15%. Although immune checkpoint inhibitors has made great progress in curing the EC, the response to programmed cell death protein 1 inhibitors are different between the ESCC and the EAC. Compared with the EAC, the ESCC has a distinct molecular makeup. There is an urgently demand to develop specific biomarkers for diagnosis and candidate therapeutic target drug to treat the disease. RNA epitranscriptomics is a new frontier of studying RNA modifications and N6-methyladenosine (m6A) is essential in various bioprocess in eukaryotic messenger RNAs (mRNAs). Accumulating researches show that m6A methylation has enormous impact on kinds of diseases. Unfortunately, the underlying mechanisms of m6A methylation in a variety of diseases have not been fully elucidated. In this review, the mechanisms of m6A modifications in the EC are sufficiently discussed, especially on proliferation, apoptosis, cell cycle and therapeutic strategies. Moreover, we will investigate the realistic clinical practice of m6A and the possibilities of capitalizing on m6A for EC early diagnosis and treatment.
RNA methylation
RNA methylation plays a necessary role in kinds of biological processes, such as gene expression, cellular differentiation and so on. With the development of detection methods in RNA modification, various types of RNA methylation were found, including N6-methyladenosine (m6A), N1-methyladenosine (m1A), N7-methylguanosine (m7G) and 5-methylcytosine (m5C)[4]. RNA methylation occurs in different RNA types. m5C has been found in mRNA, rRNA and tRNA. m7G modification includs transfer RNA(tRNA), ribosomal RNA(rRNA), messenger RNA(mRNA), and MicroRNA(miRNA)[5]. Increasing evidence supports the notion that dysregulation of RNA methylation contributes to a diverse range of human diseases, including cancer and immune system disorders.
Regulators of m6A
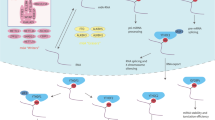
m6A modification influences various biological functions as either inhibitor or facilitator in tumor cells reversibly and dynamically. m6A can be catalyzed by RNA methyltransferase (writer), cleared off by the demethylase (eraser) and then interacts with m6A-binding protein (reader). In this part, we are going to elaborate the role of m6A in the occurrence and progression of tumors. Figure 1 visually presents the molecular mechanism of the m6A modification.
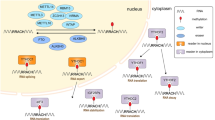
m6A writers
The m6A writers are composed of methyltransferase like protein 3 (METTL3), methyltransferase like protein 14 (METTL14), Wilm’s tumor-associated protein 1 (WTAP), zinc finger CCCH domain protein 13 (ZC3H13), RNA binding motif protein 15 (RBM15), the human homologous gene of Drosophila VIR protein (KIAA1429 or VIRMA) and as well as zinc finger protein 217 (ZFP217) and HAKAI [6,7,8,9,10]. In the combination of METTL3 and METTL14, METTL3 acts as the catalytically subunit while METTL14 plays a role of stabilizer of the complex. The complex has a binding position for S-adenosylhomocysteine (SAH) or S-adenosylmethionine (SAM) [11]. The core ingredient of the m6A methyltransferase is METTL3. WTAP does not possess any catalytic domains and has no catalytic activity on METTL3-METTL14 complex. WTAP serves as a regulator of the METTL3-METTL14 complex and markedly influence the m6A modification depositions. The targeted adenosine residues subunit was methylated following the binding of METTL3-METTL14-WTAP complex and mRNA [12, 13].
Recent year, other proteins have been identified as m6A writers. Yue et al. reported VIRMA mediated selective methylations of mRNA in 3’UTR and near the stop codon by attracting the components METTL3/METTL14/WTAP complex [14]. KIAA1429 deletion could generate aberrant RNA metabolism in folliculogenesis and the maintenance of oocyte competence [15]. KIAA1429 mediates m6A of GATA-binding protein 3 (GATA3) that further regulates cell proliferation and metastasis in liver cancer. In 2016, Patil et al., for the first time, found that RBM15 and RBM15B lead to m6A formation at specific sites of the lncRNA X-inactive specific transcript (XIST) by recruiting the m6A-methylation(m6Am) complex [16]. We also known that ZC3H13 is an m6A writer interacting with an m6A conserved combination including WTAP, Virilizer and HAKAI. And ZC3H13 translocates this compound to the cell nucleus to accelerate m6A methylation [17]. HAKAI is a necessary element required for the diverse subunits of the methyltransferase complex and recruit the core components to promote m6A deposition [18]. Zinc finger protein 217 (ZFP217), up-regulated in kinds of human tumors, is a significant oncogenicity effector [19,20,21,22]. Francesca et al. demonstrated that ZFP217 reacted with METTL3 to modulate the m6A deposition [9]. A previous publication revealed that ZFP217 activated m6A demethylase fat mass and obesity-associated protein (FTO) to coordinate m6A mRNA methylation in an m6A-YTHDF2-dependent manner[23].
Recently, newly discovered m6A RNA methyltransferases contains methyltransferase like proteins 5 and 16 (METTL5, METTL16), both can mediate m6A on specific sites of RNAs [24, 25]. Previous studies have found that METTL5 includes a SAM-binding GxGxG motif and a m6A-catalyzing NPPF motif. Bowen et al. found that METTL5 could catalyze m6A modification on the 18S rRNA around region A1832 by means of high-performance liquid chromatography-mass spectrometry (HPLC–MS) analyses [26]. Jessica et al. identified the METTL16 by mass spectrometry, suggesting a hypothetical RNA methyltransferase. METTL16 can modify m6A on U6 snRNA, kinds of ncRNAs, numerous long non-coding RNAs (lncRNAs) and pre-mRNAs [27, 28]. Lately, zinc finger CCHC domain-containing protein 4 (ZCCHC4) was discovered as a novel methyltransferase that could mainly methylate human 28S rRNA and a subset of mRNAs [29].
m6A erasers
The m6A erasers consist of Fat Mass and Obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5) which mainly remove m6A transcriptional modification within the nucleus. Thomas et al. first found FTO mediated Fe(II)- and 2OG-dependent nucleic acid demethylation [30]. The crystal structure of FTO protein has two important domains including AlkB-like domain and carboxy-terminal domain. These two domains that interacting with each other corporately catalyze FTO demethylation of 3-methylthymidine (3-meT) in single-stranded DNA and 3-methyluracil (3-meU) in single-stranded RNA [31, 32]. Jia et al. found altering expression level of FTO would change the amounts of m6A [33]. However, the position of FTO as a mainly eraser of m6A has been questioned. Jan et al. found that m6Am is dynamically and reversibly demethylated by FTO and FTO prefer to demethylate substrate of m6Am rather than m6A [34]. Nonetheless, latest study demonstrated that the distribution of FTO in the nucleus and cytoplasm is distinguishing and further affect the way of FTO influencing on kinds of RNA substrates. FTO can bind various genre of RNAs and then mediate m6A or m6Am demethylation on mRNA or small nuclear RNA (snRNA) as well as m1A in tRNA [35]. Relier et al. reported that FTO influenced stem-like properties of colorectal cancer cell through regulating cytoplasmic m6Am demethylation [36]. It seems that FTO is inclined to targets nuclear m6A and cytoplasmic m6A and m6Am in mRNA. Numerous studies demonstrated that FTO could embellish multiple kinds of RNAs, such as lncRNAs, circular RNAs (circRNAs) and mRNAs [37,38,39]. FTO firstly recognized to be a regulator in obesity and diabetes, performances different functions in cancers. As an m6A demethylase, FTO could regulate multiple functions in cancers, such as cell cycle, apoptosis, proliferation, migration, invasion, stem cell self-renewal and so on. Further studies are needed to explored the detailed functions and concrete mechanisms of FTO. FTO and ALKBH5 demethylases both pertain to the AlkB subfamily of the Fe(II)/2-oxoglutarate (2OG) dioxygenase superfamily. However, ALKBH5 expression selectively demethylates m6A, but not m6Am, indicating that ALKBH5 appears preference for m6A not for m6Am [34]. ALKBH5 reveals a stricter substrate preference and only catalyzes demethylation of m6A containing ssRNAs and the sequence of (Pu[G > A] m6AC [A/C/U]) rather than random sequences [40]. ALKBH5 level is high-expression in various cancer tissues, such as lung cancer and epithelial ovarian cancer [41, 42] but down-regulated in hepatocellular carcinoma, colon cancer and bladder cancer cells [43,44,45]. In immunotherapy, ALKBH5 has been used to regulate lactate concentration and immune cell accumulation in tumor micro-environment and change the anti-PD-1 therapeutic effect [46]. ALKBH5 not only plays a great role in cancers but also is critical for non-cancer diseases. Li et al. reported that ALKBH5 was a negative regulator in osteogenic differentiation of mesenchymal stem cells [47]. Recently, Zhou et al. proved that ALKBH5 had impact on the pathogenicity of CD4 + T cells during autoimmunity [48] and Zhao et al. demonstrated that ALKBH5 had a adverse effect on post-ischemic angiogenesis [49]. Further investigations should be performed to elucidate the relationship between ALKBH5 and human diseases.
m6A readers
Currently the well studied “readers” are YTH domain-containing proteins, including YTHDF1-3 and YTHDC1-2. In addition to the YTH reader proteins, there are some other binding proteins also stabilize m6A methylation and generate functional signal, including eukaryotic translation initiation factor 3 (eIF3), staphylococcal nuclease domain-containing 1 (SND1), heterogeneous nuclear ribonucleoprotein C (HNRNPC), heterogeneous nuclear ribonucleoprotein G(HNRNPG), heterogeneous ribonucleoprotein A2/B1(HNRNPA2B1) and insulin-like growth factor 2 mRNA binding protein 1-3 (IGF2BP1-3). Of YTH domain-containing proteins in cancer, YTHDF2 is the firstly identified reader protein and the most extensively studied. YTHDF2 consists of a P/Q/N-rich N terminus and a RNA-binding domain C terminus. C-terminal YTD domain leads to YTHDF2 binding to m6A of target mRNA and N-terminal domain leads to YTHDF2 accelerating the degradation of the targeted mRNA [50]. Notably, the latest study indicated that YTHDF2 was upregulated in prostate cancer and mediated tumor suppressors LHPP and NKX3-1 mRNA degradation in m6A-dependent way [51]. Hou et al. found YTHDF2 expression level is associated with clinical outcome and YTHDF2 deletion promotes hepatocellular carcinoma (HCC) growth, tumor angiogenesis and distant metastasis [52]. YTHDF2 can stabilize MYC mRNA of cancer stem cells and it might be a potential target to regulate MYC signaling pathway in glioblastoma [53]. Human YTHDF1 will promote protein synthesis via interacting with post-transcriptional translation mechanisms. The relationship between YTHDF1 and eIF3 contribute to the link of YTHDF1 with the translation initiation complex and YTHDF1 can bind to specific m6A sites locateing at 3′UTRs, coding regions and 5′UTRs of mRNA to promote translation [54]. According to the Oncomine database, YTHDF1 was highly expression in tumors and it overall expression level was strong correlation with survival prognosis, tumor mutational burden (TMB), immune checkpoints (ICP), microsatellite instability (MSI) and neoantigens formation. Moreover, YTHDF1 also regulates tumor microenvironment (TME) and participates in immune regulation. We can trust that YTHDF1 might be a novel moderator in tumor immunotherapy [55]. The IGF2 mRNA-binding protein family (IGF2BPs) consists of two RNA-recognition motifs (RRM) and four KH domains guiding the cytoplasmic fate of various target mRNAs and controls essential cellular functions [56, 57]. Xie et al. found that circPTPRA could hinder the recognition of m6A-modified RNAs mediatd by IGF2BP1 through interacting with KH domains of IGF2BP1. Ectopic expression of circPTPRA will inhibit the facilitation of IGF2BP1 toward cell proliferation, migration and invasion of bladder cancer cells [58]. Recent publication indicated that IGF2BP2 induced the expression of ErbB2 by maintaining the m6A sites of YAP which further affected the cell cycle and cell apoptosis of colorectal cancer cell [59]. Based on the data of bio-informatics and experimental methods, IGF2BP3 is over-expressed in bladder cancer and was strongly correlated with patients’ survival prognosis. In addition, IGF2BP3 will activate the JAK/STAT pathway and then accelerate cell proliferation [60]. On conclusion, IGF2BP3 might be a potential therapeutic target of bladder cancer that can greatly change the prognosis of patients.
Role of m6A modification in the EC
In 2019, Liu et al. found that FTO expression level was stronger in ESCC than that in compared normal tissues and the patients with higher expression of FTO frequently had worse prognosis. The western blotting indicated that FTO interacted with MMP13 and further study found FTO played oncogenic role needed stability expression of MMP13 [61]. Subsequent studies discovered that YTHDC2 expression level was lower in ESCC based on RNA-seq data consisting with TCGA database. Rs2416282 in the promoter of YTHDC2 might be a risk factor of ESCC. Knockdown YTHDC2 could enhance cell growth while elevated YTHDC2 expression could suppress cell proliferation, suggesting that YTHDC2 acts as a tumor suppressor in ESCC [62]. LINC00278 encoding Yin Yang 1 (YY1)-binding micropeptide (YY1BM) is a Y-linked lncRNA downregulated in ESCC. Cigarette smoking might decrease m6A modification sites of LINC00278 and synthesis of YY1BM. The translation efficiency of YY1BM could be enhanced by recruiting the translation machinery resulted from YTHDF1 binding with LINC00278. Mutual effection between YY1 and androgen receptor (AR) was suppressed by YY1BM and further induced the decreasing the eEF2K. Nevertheless, down-regulating YY1BM could confer ESCC more ability to nutrient deprivation through up-regulating eEF2K expression [63]. Nagaki et al. found ALKBH5 was correlated with poor prognosis in high expression level of ESCC patients demonstrating ALKBH5 might be a independent prediction factor of the survival ration. Cell cycle of ESCC cells will be delayed and accumulated to G0/G1 phase by knocking down ALKBH5. This resulted in suppression of the ESCC biological behaviour, such as proliferation and migration [64]. The IHC results showed that the higher expression of METTL3 was associated with the worse survival. Further studies found the METTL3 was positively correlated with the unfavourable survival indicating METTL3 might act as a symbol of disease-free survival (DFS) and overall survival (OS) [65]. Recently, interests focusing on function of non-coding RNA in EC became a hot topic. The underlying mechanism of miR-193a-3p has been uncovered that researchers find miR-193-3p suppresses the expression of ALKBH5. Furthermore, the miR-193-3p-ALKBH5 coupled loop could enhance cell invasiveness in vitro experiment [66]. In 2021, with the popularization of methylated RNA immunoprecipitation sequencing (MeRIP-Seq), researchers found METTL3 could affect glutaminase 2 (GLS2) via m6A modification. Chen et al. demonstrated that a direct strong binding interaction between GLS2 and METTL3 was detected using a RIP assay. They also discovered that knock-down of GLS2 expression level inhibited the invasiveness and migration capability of ESCC cells, indicating that GLS2 might be a carcinogenic risk in ESCC [67]. Wang et al. revealed a previously unknown mechanism that YTHDF/METTL3 together regulating m6A of APC mRNA would reduced APC expression. Promotion of β-catenin signaling pathway and accumulation of aerobic glycolysis have been confirmed through the aforementioned mechanism. The mechanism of METTL3/YTHDF coupled m6A regulation toward APC mRNA may provide therapeutic strategy for ESCC patients [68]. Hou et al. found that METTL3 could affect AKT signaling pathway and then alter the biological behavior of the EC [69]. Earlier studies found Colon cancer-associated transcript 2 gene (CCAT2) was high-expression in tumor and was associated with patient’s survival prognosis [70]. Wu et al. recently discovered CCAT2 could reduce miR-200b expression and further lead to IGF2BP2 high-expression. In turn, IGF2BP2 enhanced TK1 stability by identifying its m6A sites. On conclusion, this study proved that CCAT2 alleviate its suppression effects on IGF2BP2 by binding to miR-200b, resulting in upregulation of TK1 expression level and facilitation of ESCC [71]. Cellular lipid synthesis was inhibited by down-regulation of HNRNPA2B1 induced decreasing of fatty acid synthesis enzymes ACLY and ACC1. The result is that HNRNPA2B1 as a potential oncogenic factor could affect cell biological behaviour of ESCC [72]. Another researcher reported that HNRNPA2B1 was a prognosis predict factor of EC by influencing the miR-17-92 cluster [73]. AS for HNRNPC, HNRNPC can strengthen the stability of ZEB1 mRNA and ZEB1-induced high-expression of LBX2-AS1 enhanced migration and epithelial-mesenchymal transition capability of ESCC [74]. Currently, majority of investigations on m6A in the EC focused on the ESCC, but seldom on the EAC. Lately, we can see a lot of studies uncovering the mechanism about m6A and adenocarcinoma. And more exploration is needed in studying the relationship between EAC and m6A modification.
The diagnosis potential of m6A for the EC
Early time, METTL3 up-regulated in tumor tissues was negatively related with patients’ DFS and OS. Liu et al. found 18F-FDG intake was increased along with high expression level of METTL3. The underlying mechanism of METTL3 increasing the intake of 18F-FDG might be controlled by GLUT1 and HK2. So PET/CT is a noninvasive monitoring to assess the condition of METTL3 [75]. Maybe we can use PET/CT and METTL3 coupled indicators as predictors of EC. Li et al. constructed a 4-miRNA survival predict model by combining the data of TCGA with GEO datasets [76]. With the in-depth of m6A research, m6A modification is expected to be an early detection biomarkers of the EC.
The therapeutic potential of m6A for the EC
The traditional treatment modalities for the EC include operative treatment and radiotherapy as local therapies, while chemotherapy and targeted drug therapy as systemic treatments. In early stage of EC, a combination of endoscopic resection and adjuvant chemoradiotherapy is a therapeutic option with promising results. As for advanced ESCC, the aim of the treatment is to alleviate patient’s symptoms and prolong patient’s survival, therefore, chemotherapy and radiotherapy are usually adopted. The potential applications of targeted treatment based on m6A was concluded in previous studies. MO-I-500, a selected inhibitor of FTO, inhibited survival and colony formation of breast cancer cell lines SUM149-MA [77]. Metabolic reprogramming, driven by IGF2BP3, facilitates the development of acquired resistance to EGFR inhibitors in non-small cell lung cancer which suggests a novel perspective on the alteration of drug resistance [78]. Yankova et al. found treating tumours with STM2457, a METTL3 inhibitor, lead to reduced acute myeloid leukaemia (AML) growth and an increase in differentiation and apoptosis [79]. Other researchers also found that STM2457 can reverse small cell lung cancer chemoresistance by inducing mitophagy [80]. Anti-HIV Drug Elvitegravir enhanced the ubiquitination degradation of METTL3 by promoting the interaction between METTL3 and E3 ubiquitin ligase STUB1, which confirmed that the drug has a significant inhibitory effect on ESCC invasion and metastasis [81]. However, the development of inhibitors or activators of m6A-related proteins is still in the initial stage. Existing inhibitors or activators of m6A-related proteins generally have problems such as low activity, poor specificity, over-complex phenotype of intracellular effects. Therefore, m6A-related protein inhibitors or activators are mainly used in preclinical experimental studies and there is no researches applying inhibitors or activators of m6A-related proteins on ESCC patients. Some researches had reported that m6A overall expression level was up-regulated after Platinum-based chemotherapy. Platinum could induce SNHG3 expression but suppress microRNA-186-5p. The result is that m6A level is promoted by SNHG3 but inhibited by miR-186-5p through targeting METTL3. Zhang et al. put forward a hypothesis that regulating the overall m6A expression level might be an innovative strategy to improve the platinum treatment efficacy in EC patients [82]. Immune checkpoint therapy is a milestone in the field of cancer treatment. Through targeting PD-1 or PD-L1, the adaptive immune system can eliminate cancer cells and achieve a promising therapeutic efficacy. It is reported that FTO could promote melanoma tumorigenesis and decrease its response to immunotherapy in melanoma [83]. In recent years, immunotherapy achieved exactly clinical benefits in patients with ESCC. Guo et al. found that m6A influenced the micro-circumstances of tumor-infiltrating immune cells, suggesting that m6A could change the tumor immune microenvironment (TIME) [84]. Hence, m6A modification regulators is expected to elevate the efficacy of immunotherapy. Taken together, m6A methylation regulator not only adjust the PD-L1 expression level but also regulate the situation of immune cell infiltration. Therefore, a prognostic model including five m6A control elements (HNRNPC, RBM15, IGF2BP3, METTL16 and KIAA1429) was constructed to predict the prognosis of ESCC. From above researches, we hope that the m6A modification regulators may elevate the effect of immunotherapy.
Discussion
Currently, the main treatments of the EC include surgery, radiotherapy, chemotherapy, target therapy and Immune checkpoint therapy. However, advanced esophageal carcinoma with high occurrences of metastasis and the overall survival is disappointment. It is crucial to clarify the specific mechanism of EC carcinogenesis. m6A modification is a new way of RNA manifestation. With the development of m6A profiling technologies, it is hoped that the pace of discovering the location of m6A modification will greatly accelerate. The m6A methylation modification has drawn more and more vision. Previous studies had explicit the mechanism of m6A modification in human cancer occurrence and development in kinds of cancer types such as liver cancer and non-small-cell lung cancer etc. In this research, the initiation and progression of the EC are highly associated with kinds of m6A regulators. METTL3 is not only up-regulated in the EC but also associated with a patient's DFS and OS. Nevertheless, ALKBH5 might act as a suppressor gene towards the EC, and patients with a lower expression level of ALKBH5 is usually accompanied by poor prognosis. Esophageal cancer tissues always with high-expression of HNRNPC and was correlated with poor prognosis of survival. From the above findings, we know that m6A methylation modification can regulate the rate of cell proliferation, affect distant of tumor cell metastasis, change ability of cancer cell invasion and alter the drug resistance of tumor cell.
The m6A regulators have the potential to be clinical treatment targets for different types of cancers. Rhein derived from the rhizome of Rheum palmatum was found to competitively bind to the specific FTO site [85]. R-2-hydroxyglutarate (R-2HG), produced by mutant isocitrate dehydrogenase 1/2 (IDH1/2) enzymes, exhibits a extensive anti-leukemic activity by inhibiting FTO activity and increasing m6A RNA modification in R-2HG-sensitive leukemia cells [86]. However, drug resistance or decreasing sensitivity to radiotherapy continues to be a dominant barrier to remedial treatment, leading to treatment failure and tumour progression. Su et al. found YTHDC1 regulates PTEN/PI3K/AKT signalling pathway and plays a critical role in cisplatin resistance of bladder cancer [87]. In addition, METTL3 improves the progression of pancreatic ductal adenocarcinoma and resistance to gemcitabine by modifying m6A of DDX23 mRNA [88]. Based on the literature above, we can see that m6A is very promising for enhancing drug resistance. NRP1, a transmembrane glycoprotein, contributes to stemness and radioresistance of breast cancer through WTAP-mediated m6A methylation of Bcl-2 mRNA [89]. Wu et al. discovered that METTL3 mediating the m6A methylation of circCUX1, a specific circRNA, lead radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway [90]. These articles provide new clues about the therapeutic direction of m6a in radiotherapy (Table 1).
Previous studies have validated that the m6A score could be a credible biomarker for predicting the efficacy of anti-PD-1/L1 immunotherapy in different types of tumours such as melanoma, breast cancer and colorectal cancer. In melanoma, lower m6Ascores always correlated with more sensitive anti-PD-1 and anti-CTLA4 treatment responses [91]. Some researchers have confirmed that the specific suppression of METTL3 myeloids could attenuate the inhibitory treatment of PD-1 by affecting the reprogramming of macrophages [92]. The potential mechanism of m6A in the immunotherapy of esophageal cancer remains unclear. Checkmate577, an adjuvant nivolumab therapy research, demonstrates for the first time that adjuvant immune checkpoint inhibitors therapy can lead to clinically significant improvements in disease-free survival in patients with resectable esophageal cancer and gastroesophageal junction cancer [93].This study serves as a precursor to the implementation of immunotherapy in the treatment of esophageal cancer. The prognostic survival of patients is closely associated with the tumor immune microenvironment. Nie et al. used single-cell mapping and immune infiltration risk model to estimate the m6A epigenetic-based riskscore [94]. They found ESCC patients with higher risk scores have lower expression levels of major immune cells such as in natural killer T cell (NKT), CD4 + naive T cells, M1 macrophages, ADC, and macrophages. METTL3 could regulate esophageal cancer proliferation, invasion and immunity via the downstream target IFIT2. TIMER database revealed a significant correlation between the expression of METTL3 and the degree of infiltration by B cells and macrophages [95]. The mechanism of mettl3 in the immunotherapy of esophageal cancer needs to be further explored. Moreover, Paclitaxel sensitivity is lower in the mutant CSMD1 group and CSMD1 mutation is associated with tumor invasion of immune cells with more follicular helper T cells and fewer resting state dendritic cells. This phenomenon suggests that CSMD1 mutation may serve as an immune-related biomarker for predicting the ESCC patient’s prognosis and treatment response to paclitaxel [96].m6A modification study progressing is still at the initial stage and there are still many challenges. The drug targeting specific m6A proteins is needed to design via high throughput drug screening and structural studies. We hope that m6A modification inhibitors will become a promising therapeutic methods for patients suffering different cancers. Scientists are devoted to inventing m6A modification detection kit for specific cancer to diagnosis in its early stage and developing particular reagent targeting m6A proteins to obtain good therapeutic effect.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Prabhu A, Obi KO, Rubenstein JH (2014) The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol 109(6):822–827. https://doi.org/10.1038/ajg.2014.71
Coleman HG, Xie SH, Lagergren J (2018) The epidemiology of esophageal adenocarcinoma. Gastroenterology 154(2):390–405. https://doi.org/10.1053/j.gastro.2017.07.046
Cao J, Shu X, Feng XH, Liu J (2021) Mapping messenger RNA methylations at single base resolution. Curr Opin Chem Biol 63:28–37. https://doi.org/10.1016/j.cbpa.2021.02.001
Song H, Zhang J, Liu B, Xu J, Cai B, Yang H, Straube J, Yu X, Ma T (2021) Biological roles of RNA m5C modification and its implications in Cancer immunotherapy. Biomark Res 10(1):15. https://doi.org/10.1186/s40364-022-00362-8
Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, Yan X, Liao H, Chen X, Xie K, Li J, Liao M, Huang J, Yuan K, Zeng Y, Wu H (2019) KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer 18(1):186. https://doi.org/10.1186/s12943-019-1106-z
Wang X, Tian L, Li Y, Wang J, Yan B, Yang L, Li Q, Zhao R, Liu M, Wang P, Sun Y (2021) RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res 40(1):80. https://doi.org/10.1186/s13046-021-01871-4
Gong PJ, Shao YC, Yang Y, Song WJ, He X, Zeng YF, Huang SR, Wei L, Zhang JW (2020) Analysis of N6-methyladenosine methyltransferase reveals METTL14 and ZC3H13 as tumor suppressor genes in breast cancer. Front Oncol 10:578963. https://doi.org/10.3389/fonc.2020.578963
Aguilo F, Zhang F, Sancho A, Fidalgo M, Di Cecilia S, Vashisht A, Lee DF, Chen CH, Rengasamy M, Andino B, Jahouh F, Roman A, Krig SR, Wang R, Zhang W, Wohlschlegel JA, Wang J, Walsh MJ (2015) Coordination of m(6)A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell 17(6):689–704. https://doi.org/10.1016/j.stem.2015.09.005
Bawankar P, Lence T, Paolantoni C, Haussmann IU, Kazlauskiene M, Jacob D, Heidelberger JB, Richter FM, Nallasivan MP, Morin V, Kreim N, Beli P, Helm M, Jinek M, Soller M, Roignant JY (2021) Hakai is required for stabilization of core components of the m6A mRNA methylation machinery. Nat Commun 12(1):3778. https://doi.org/10.1038/s41467-021-23892-5
Zeng C, Huang W, Li Y, Weng H (2020) Roles of METTL3 in cancer: mechanisms and therapeutic targeting. J Hematol Oncol 13(1):117. https://doi.org/10.1186/s13045-020-00951-w
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10(2):93–95. https://doi.org/10.1038/nchembio.1432
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, Zhao X, Li A, Yang Y, Dahal U, Lou XM, Liu X, Huang J, Yuan WP, Zhu XF, Cheng T, Zhao YL, Wang X, Rendtlew Danielsen JM, Liu F, Yang YG (2014) Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24(2):177–189. https://doi.org/10.1038/cr.2014.3
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, Wang F, Wang X, Shen B, Wang Y, Feng X, He C, Liu J (2018) VIRMA mediates preferential m6A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4:10. https://doi.org/10.1038/s41421-018-0019-0
Hu Y, Ouyang Z, Sui X, Qi M, Li M, He Y, Cao Y, Cao Q, Lu Q, Zhou S, Liu L, Liu L, Shen B, Shu W, Huo R (2020) Oocyte competence is maintained by m6A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ 27(8):2468–2483. https://doi.org/10.1038/s41418-020-0516-1
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR (2016) m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537(7620):369–373. https://doi.org/10.1038/nature19342
Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, Lan F, Shi YG, He C, Shi Y, Diao J (2018) Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell 69(6):1028-1038.e6. https://doi.org/10.1016/j.molcel.2018.02.015
Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, Eeckhout D, El-Showk S, Li H, Zhong S, De Jaeger G, Mongan NP, Hejátko J, Helariutta Y, Fray RG (2017) Identification of factors required for m6 A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol 215(1):157–172. https://doi.org/10.1111/nph.14586
Lee DF, Walsh MJ, Aguiló F (2016) ZNF217/ZFP217 meets chromatin and RNA. Trends Biochem Sci 41(12):986–988. https://doi.org/10.1016/j.tibs.2016.07.013
Northey JJ, Barrett AS, Acerbi I, Hayward MK, Talamantes S, Dean IS, Mouw JK, Ponik SM, Lakins JN, Huang PJ, Wu J, Shi Q, Samson S, Keely PJ, Mukhtar RA, Liphardt JT, Shepherd JA, Hwang ES, Chen YY, Hansen KC, Littlepage LE, Weaver VM (2020) Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J Clin Invest 130(11):5721–5737. https://doi.org/10.1172/JCI129249
Si W, Zhao Y, Zhou J, Zhang Q, Zhang Y (2019) The coordination between ZNF217 and LSD1 contributes to hepatocellular carcinoma progress and is negatively regulated by miR-101. Exp Cell Res 379(1):1–10. https://doi.org/10.1016/j.yexcr.2019.03.017
Li P, Maines-Bandiera S, Kuo WL, Guan Y, Sun Y, Hills M, Huang G, Collins CC, Leung PC, Gray JW, Auersperg N (2007) Multiple roles of the candidate oncogene ZNF217 in ovarian epithelial neoplastic progression. Int J Cancer 120(9):1863–1873. https://doi.org/10.1002/ijc.22300
Song T, Yang Y, Wei H, Xie X, Lu J, Zeng Q, Peng J, Zhou Y, Jiang S, Peng J (2019) Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res 47(12):6130–6144. https://doi.org/10.1093/nar/gkz312
van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR, Graille M, Lafontaine DLJ (2019) The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res 47(15):7719–7733. https://doi.org/10.1093/nar/gkz619
Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK (2017) The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169(5):824-835.e14. https://doi.org/10.1016/j.cell.2017.05.003
Rong B, Zhang Q, Wan J, Xing S, Dai R, Li Y, Cai J, Xie J, Song Y, Chen J, Zhang L, Yan G, Zhang W, Gao H, Han JJ, Qu Q, Ma H, Tian Y, Lan F (2020) Ribosome 18S m6A methyltransferase METTL5 promotes translation initiation and breast cancer cell growth. Cell Rep. 33(12):108544. https://doi.org/10.1016/j.celrep.2020.108544
Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA (2016) Methyltransferase-like protein 16 binds the 3’-terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci USA 113(49):14013–14018. https://doi.org/10.1073/pnas.1614759113
Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, Sloan KE, Bohnsack MT (2017) Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18(11):2004–2014. https://doi.org/10.15252/embr.201744940
Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, Chen K, Lu Z, Chen H, Shi YG, Lan F, Fan J, Klaholz BP, Pan T, Shi Y, He C (2019) N6-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol 15(1):88–94. https://doi.org/10.1038/s41589-018-0184-3
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O’Rahilly S, Schofield CJ (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318(5855):1469–72. https://doi.org/10.1126/science.1151710
Han Z, Niu T, Chang J, Lei X, Zhao M, Wang Q, Cheng W, Wang J, Feng Y, Chai J (2010) Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature 464(7292):1205–1209. https://doi.org/10.1038/nature08921
Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou Z, He C (2008) Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett 582(23–24):3313–3319. https://doi.org/10.1016/j.febslet.2008.08.019
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7(12):885–887. https://doi.org/10.1038/nchembio.687
Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, Gross SS, Elemento O, Debart F, Kiledjian M, Jaffrey SR (2017) Reversible methylation of m6Am in the 5’ cap controls mRNA stability. Nature 541(7637):371–375. https://doi.org/10.1038/nature21022
Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, Shi H, Cui X, Su R, Klungland A, Jia G, Chen J, He C (2018) Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell 71(6):973-985.e5. https://doi.org/10.1016/j.molcel.2018.08.011
Relier S, Ripoll J, Guillorit H, Amalric A, Achour C, Boissière F, Vialaret J, Attina A, Debart F, Choquet A, Macari F, Marchand V, Motorin Y, Samalin E, Vasseur JJ, Pannequin J, Aguilo F, Lopez-Crapez E, Hirtz C, Rivals E, Bastide A, David A (2021) FTO-mediated cytoplasmic m6Am demethylation adjusts stem-like properties in colorectal cancer cell. Nat Commun 12(1):1716. https://doi.org/10.1038/s41467-021-21758-4
Mo WL, Deng LJ, Cheng Y, Yu WJ, Yang YH, Gu WD (2021) Circular RNA hsa_circ_0072309 promotes tumorigenesis and invasion by regulating the miR-607/FTO axis in non-small cell lung carcinoma. Aging (Albany NY) 13(8):11629–11645. https://doi.org/10.18632/aging.202856
Qin B, Dong M, Wang Z, Wan J, Xie Y, Jiao Y, Yan D (2021) Long non-coding RNA CASC15 facilitates esophageal squamous cell carcinoma tumorigenesis via decreasing SIM2 stability via FTO-mediated demethylation. Oncol Rep. 45(3):1059–1071. https://doi.org/10.3892/or.2020.7917
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou K, Wang L, Cao Y, Sun P, Wang T (2020) The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (Lond) 40(10):484–500. https://doi.org/10.1002/cac2.12075
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49(1):18–29. https://doi.org/10.1016/j.molcel.2012.10.015
Zhu H, Gan X, Jiang X, Diao S, Wu H, Hu J (2019) ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res 38(1):163. https://doi.org/10.1186/s13046-019-1159-2
Zhu Z, Qian Q, Zhao X, Ma L, Chen P (2020) N6-methyladenosine ALKBH5 promotes non-small cell lung cancer progress by regulating TIMP3 stability. Gene 731:144348. https://doi.org/10.1016/j.gene.2020.144348
Yan G, An Y, Xu B, Wang N, Sun X, Sun M (2021) Potential impact of ALKBH5 and YTHDF1 on tumor immunity in colon adenocarcinoma. Front Oncol 11:670490. https://doi.org/10.3389/fonc.2021.670490
Yu H, Yang X, Tang J, Si S, Zhou Z, Lu J, Han J, Yuan B, Wu Q, Lu Q, Yang H (2020) ALKBH5 inhibited cell proliferation and sensitized bladder cancer cells to cisplatin by m6A-CK2α-mediated glycolysis. Mol Ther Nucleic Acids 22(23):27–41. https://doi.org/10.1016/j.omtn.2020.10.031
Chen Y, Zhao Y, Chen J, Peng C, Zhang Y, Tong R, Cheng Q, Yang B, Feng X, Lu Y, Xie H, Zhou L, Wu J, Zheng S (2020) ALKBH5 suppresses malignancy of hepatocellular carcinoma via m6A-guided epigenetic inhibition of LYPD1. Mol Cancer 19(1):123. https://doi.org/10.1186/s12943-020-01239-w
Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, Gonzalez GM, Wang Y, Patel SP, Rana TM (2020) ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci USA 117(33):20159–20170. https://doi.org/10.1073/pnas.1918986117
Li Z, Wang P, Li J, Xie Z, Cen S, Li M, Liu W, Ye G, Zheng G, Ma M, Wang S, Yu W, Wu Y, Shen H (2021) The N6-methyladenosine demethylase ALKBH5 negatively regulates the osteogenic differentiation of mesenchymal stem cells through PRMT6. Cell Death Dis 12(6):578. https://doi.org/10.1038/s41419-021-03869-4
Zhou J, Zhang X, Hu J, Qu R, Yu Z, Xu H, Chen H, Yan L, Ding C, Zou Q, Ye Y, Wang Z, Flavell RA, Li HB (2021) m6A demethylase ALKBH5 controls CD4+ T cell pathogenicity and promotes autoimmunity. Sci Adv 7(25):eabg0470. https://doi.org/10.1126/sciadv.abg0470
Zhao Y, Hu J, Sun X, Yang K, Yang L, Kong L, Zhang B, Li F, Li C, Shi B, Hu K, Sun A, Ge J (2021) Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin Transl Med 11(5):e402. https://doi.org/10.1002/ctm2.402
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505(7481):117–120. https://doi.org/10.1038/nature12730
Li J, Xie H, Ying Y, Chen H, Yan H, He L, Xu M, Xu X, Liang Z, Liu B, Wang X, Zheng X, Xie L (2020) YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer 19(1):152. https://doi.org/10.1186/s12943-020-01267-6
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, Hu B, Zhou J, Zhao Z, Feng M, Zhang H, Shen B, Huang X, Sun B, Smyth MJ, He C, Xia Q (2019) YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer 18(1):163. https://doi.org/10.1186/s12943-019-1082-3
Dixit D, Prager BC, Gimple RC, Poh HX, Wang Y, Wu Q, Qiu Z, Kidwell RL, Kim LJY, Xie Q, Vitting-Seerup K, Bhargava S, Dong Z, Jiang L, Zhu Z, Hamerlik P, Jaffrey SR, Zhao JC, Wang X, Rich JN (2021) The RNA m6A reader YTHDF2 maintains oncogene expression and is a targetable dependency in glioblastoma stem cells. Cancer Discov 11(2):480–499. https://doi.org/10.1158/2159-8290.CD-20-0331
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C (2015) N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161(6):1388–1399. https://doi.org/10.1016/j.cell.2015.05.014
Hu J, Qiu D, Yu A, Hu J, Deng H, Li H, Yi Z, Chen J, Zu X (2021) YTHDF1 is a potential pan-cancer biomarker for prognosis and immunotherapy. Front Oncol 11:607224. https://doi.org/10.3389/fonc.2021.607224
Korn SM, Ulshöfer CJ, Schneider T, Schlundt A (2021) Structures and target RNA preferences of the RNA-binding protein family of IGF2BPs: an overview. Structure 29(8):787–803. https://doi.org/10.1016/j.str.2021.05.001
Wächter K, Köhn M, Stöhr N, Hüttelmaier S (2013) Subcellular localization and RNP formation of IGF2BPs (IGF2 mRNA-binding proteins) is modulated by distinct RNA-binding domains. Biol Chem 394(8):1077–1090. https://doi.org/10.1515/hsz-2013-0111
Xie F, Huang C, Liu F, Zhang H, Xiao X, Sun J, Zhang X, Jiang G (2021) CircPTPRA blocks the recognition of RNA N6-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer 20(1):68. https://doi.org/10.1186/s12943-021-01359-x
Cui J, Tian J, Wang W, He T, Li X, Gu C, Wang L, Wu J, Shang A (2021) IGF2BP2 promotes the progression of colorectal cancer through a YAP-dependent mechanism. Cancer Sci. https://doi.org/10.1111/cas.15083
Huang W, Li Y, Zhang C, Zha H, Zhou X, Fu B, Guo J, Wang G (2020) IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J Cell Mol Med 24(23):13949–13960. https://doi.org/10.1111/jcmm.16003
Liu S, Huang M, Chen Z, Chen J, Chao Q, Yin X, Quan M (2020) FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Exp Cell Res 389(1):111894. https://doi.org/10.1016/j.yexcr.2020.111894
Yang N, Ying P, Tian J, Wang X, Mei S, Zou D, Peng X, Gong Y, Yang Y, Zhu Y, Ke J, Zhong R, Chang J, Miao X (2020) Genetic variants in m6A modification genes are associated with esophageal squamous-cell carcinoma in the Chinese population. Carcinogenesis 41(6):761–768. https://doi.org/10.1093/carcin/bgaa012
Wu S, Zhang L, Deng J, Guo B, Li F, Wang Y, Wu R, Zhang S, Lu J, Zhou Y (2020) A novel micropeptide encoded by Y-linked LINC00278 links cigarette smoking and AR signaling in male esophageal squamous cell carcinoma. Cancer Res 80(13):2790–2803. https://doi.org/10.1158/0008-5472.CAN-19-3440
Nagaki Y, Motoyama S, Yamaguchi T, Hoshizaki M, Sato Y, Sato T, Koizumi Y, Wakita A, Kawakita Y, Imai K, Nanjo H, Watanabe H, Imai Y, Minamiya Y, Kuba K (2020) m6 A demethylase ALKBH5 promotes proliferation of esophageal squamous cell carcinoma associated with poor prognosis. Genes Cells 25(8):547–561. https://doi.org/10.1111/gtc.12792
Xia TL, Yan SM, Yuan L, Zeng MS (2020) Upregulation of METTL3 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Manag Res 13(12):5729–5737. https://doi.org/10.2147/CMAR.S245019
Xue J, Xiao P, Yu X, Zhang X (2021) A positive feedback loop between AlkB homolog 5 and miR-193a-3p promotes growth and metastasis in esophageal squamous cell carcinoma. Hum Cell 34(2):502–514. https://doi.org/10.1007/s13577-020-00458-z
Chen X, Huang L, Yang T, Xu J, Zhang C, Deng Z, Yang X, Liu N, Chen S, Lin S (2021) METTL3 promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression. Front Oncol 11:667451. https://doi.org/10.3389/fonc.2021.667451
Wang W, Shao F, Yang X, Wang J, Zhu R, Yang Y, Zhao G, Guo D, Sun Y, Wang J, Xue Q, Gao S, Gao Y, He J, Lu Z (2021) METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N6-methyladenosine-dependent YTHDF binding. Nat Commun 12(1):3803. https://doi.org/10.1038/s41467-021-23501-5
Hou H, Zhao H, Yu X, Cong P, Zhou Y, Jiang Y, Cheng Y (2020) METTL3 promotes the proliferation and invasion of esophageal cancer cells partly through AKT signaling pathway. Pathol Res Pract 216(9):153087. https://doi.org/10.1016/j.prp.2020.153087
Zhang X, Xu Y, He C, Guo X, Zhang J, He C, Zhang L, Kong M, Chen B, Zhu C (2015) Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 111(7):834–839. https://doi.org/10.1002/jso.23888
Wu X, Fan Y, Liu Y, Shen B, Lu H, Ma H (2021) Long non-coding RNA CCAT2 promotes the development of esophageal squamous cell carcinoma by inhibiting miR-200b to upregulate the IGF2BP2/TK1 axis. Front Oncol 11:680642. https://doi.org/10.3389/fonc.2021.680642
Guo H, Wang B, Xu K, Nie L, Fu Y, Wang Z, Wang Q, Wang S, Zou X (2020) m6A reader HNRNPA2B1 promotes esophageal cancer progression via up-regulation of ACLY and ACC1. Front Oncol 10:553045. https://doi.org/10.3389/fonc.2020.553045
Li K, Chen J, Lou X, Li Y, Qian B, Xu D, Wu Y, Ma S, Zhang D, Cui W (2021) HNRNPA2B1 affects the prognosis of esophageal cancer by regulating the miR-17–92 cluster. Front Cell Dev Biol 9:658642. https://doi.org/10.3389/fcell.2021.658642
Zhang Y, Chen W, Pan T, Wang H, Zhang Y, Li C (2019) LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs. Biochem Biophys Res Commun 511(3):566–572. https://doi.org/10.1016/j.bbrc.2019.02.079
Liu XS, Yuan LL, Gao Y, Zhou LM, Yang JW, Pei ZJ (2020) Overexpression of METTL3 associated with the metabolic status on 18F-FDG PET/CT in patients with Esophageal Carcinoma. J Cancer 11(16):4851–4860. https://doi.org/10.7150/jca.44754
Li L, Xie R, Wei Q (2021) Network analysis of miRNA targeting m6A-related genes in patients with esophageal cancer. PeerJ 9:e11893. https://doi.org/10.7717/peerj.11893
Singh B, Kinne HE, Milligan RD, Washburn LJ, Olsen M, Lucci A (2016) Important role of FTO in the survival of rare panresistant triple-negative inflammatory breast cancer cells facing a severe metabolic challenge. PLoS One 11(7):e0159072. https://doi.org/10.1371/journal.pone.0159072
Lin Z, Li J, Zhang J, Feng W, Lu J, Ma X, Ding W, Ouyang S, Lu JJ, Yue P, Wan G, Liu P, Zhang X (2023) Metabolic reprogramming driven by IGF2BP3 promotes acquired resistance to EGFR inhibitors in non-small cell lung cancer. Cancer Res. CAN-22-3059. https://doi.org/10.1158/0008-5472.CAN-22-3059
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, Pilka ES, Aspris D, Leggate D, Hendrick AG, Webster NA, Andrews B, Fosbeary R, Guest P, Irigoyen N, Eleftheriou M, Gozdecka M, Dias JML, Bannister AJ, Vick B, Jeremias I, Vassiliou GS, Rausch O, Tzelepis K, Kouzarides T (2021) Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 593(7860):597–601. https://doi.org/10.1038/s41586-021-03536-w
Sun Y, Shen W, Hu S, Lyu Q, Wang Q, Wei T, Zhu W, Zhang J (2023) METTL3 promotes chemoresistance in small cell lung cancer by inducing mitophagy. J Exp Clin Cancer Res 42(1):65. https://doi.org/10.1186/s13046-023-02638-9
Liao L, He Y, Li SJ, Zhang GG, Yu W, Yang J, Huang ZJ, Zheng CC, He QY, Li Y, Li B (2022) Anti-HIV drug elvitegravir suppresses cancer metastasis via increased proteasomal degradation of m6A methyltransferase METTL3. Cancer Res 82(13):2444–2457. https://doi.org/10.1158/0008-5472.CAN-21-4124
Zhang M, Bai M, Wang L, Lu N, Wang J, Yan R, Cui M, Yan H, Zhang L (2021) Targeting SNHG3/miR-186-5p reverses the increased m6A level caused by platinum treatment through regulating METTL3 in esophageal cancer. Cancer Cell Int 21(1):114. https://doi.org/10.1186/s12935-021-01747-9
Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C, He YY (2019) m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun 10(1):2782. https://doi.org/10.1038/s41467-019-10669-0
Guo W, Tan F, Huai Q, Wang Z, Shao F, Zhang G, Yang Z, Li R, Xue Q, Gao S, He J (2021) Comprehensive analysis of PD-L1 expression, immune infiltrates, and m6A RNA methylation regulators in esophageal squamous cell carcinoma. Front Immunol 12:669750. https://doi.org/10.3389/fimmu.2021.669750
Chen B, Ye F, Yu L, Jia G, Huang X, Zhang X, Peng S, Chen K, Wang M, Gong S, Zhang R, Yin J, Li H, Yang Y, Liu H, Zhang J, Zhang H, Zhang A, Jiang H, Luo C, Yang CG (2012) Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc 134(43):17963–17971. https://doi.org/10.1021/ja3064149
Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, Yu M, Skibbe J, Dai Q, Zou D, Wu T, Yu K, Weng H, Huang H, Ferchen K, Qin X, Zhang B, Qi J, Sasaki AT, Plas DR, Bradner JE, Wei M, Marcucci G, Jiang X, Mulloy JC, Jin J, He C, Chen J (2018) R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell 172(1–2):90-105.e23. https://doi.org/10.1016/j.cell.2017.11.031
Su Y, Wang B, Huang J, Huang M, Lin T (2023) YTHDC1 positively regulates PTEN expression and plays a critical role in cisplatin resistance of bladder cancer. Cell Prolif. https://doi.org/10.1111/cpr.13404
Lin C, Li T, Wang Y, Lai S, Huang Y, Guo Z, Zhang X, Weng S (2023) METTL3 enhances pancreatic ductal adenocarcinoma progression and gemcitabine resistance through modifying DDX23 mRNA N6 adenosine methylation. Cell Death Dis 14(3):221. https://doi.org/10.1038/s41419-023-05715-1
Wang Y, Zhang L, Sun XL, Lu YC, Chen S, Pei DS, Zhang LS (2023) NRP1 contributes to stemness and potentiates radioresistance via WTAP-mediated m6A methylation of Bcl-2 mRNA in breast cancer. Apoptosis 28(1–2):233–246. https://doi.org/10.1007/s10495-022-01784-3
Wu P, Fang X, Liu Y, Tang Y, Wang W, Li X, Fan Y (2021) N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis 12(4):298. https://doi.org/10.1038/s41419-021-03558-2
Wang G, Zeng D, Sweren E, Miao Y, Chen R, Chen J, Wang J, Liao W, Hu Z, Kang S, Garza LA (2023) N6-methyladenosine RNA methylation correlates with immune microenvironment and immunotherapy response of melanoma. J Invest Dermatol. https://doi.org/10.1016/j.jid.2023.01.027
Yin H, Zhang X, Yang P, Zhang X, Peng Y, Li D, Yu Y, Wu Y, Wang Y, Zhang J, Ding X, Wang X, Yang A, Zhang R (2021) RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat Commun 12(1):1394. https://doi.org/10.1038/s41467-021-21514-8
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M; CheckMate 577 Investigators (2021) Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384(13):1191–1203. https://doi.org/10.1056/NEJMoa2032125
Nie Y, Yao G, Xu X, Liu Y, Yin K, Lai J, Li Q, Zhou F, Yang Z (2023) Single-cell mapping of N6-methyladenosine in esophageal squamous cell carcinoma and exploration of the risk model for immune infiltration. Front Endocrinol (Lausanne) 21(14):1155009. https://doi.org/10.3389/fendo.2023.1155009
Ge F, Li Z, Hu J, Pu Y, Zhao F, Kong L (2022) METTL3/m6A/IFIT2 regulates proliferation, invasion and immunity in esophageal squamous cell carcinoma. Front Pharmacol 20(13):1002565. https://doi.org/10.3389/fphar.2022.1002565
Fan X, Song J, Fan Y, Li J, Chen Y, Zhu H, Zhang Z (2021) CSMD1 mutation related to immunity can be used as a marker to evaluate the clinical therapeutic effect and prognosis of patients with esophageal cancer. Int J Gen Med 23(14):8689–8710. https://doi.org/10.2147/IJGM.S338284
Funding
This work was partially supported by the National Key R&D Program of China (2017YFC0113500); Major science and technology projects of Zhejiang province (2020C03058); Diagnosis and treatment technology research center of pulmonary neoplasm in Zhejiang Province(JBZX-202007); Key disciplines of traditional Chinese medicine in Zhejiang Province (2017-XK-A33).
Author information
Authors and Affiliations
Contributions
HC and TX designed the content of this review. HC contributed to the drafting and editing of the manuscript. TX assisted in the revision of the manuscript. WLM, NMQ and LJQ critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, C., Teng, X., Wang, L. et al. The implications of N6-methyladenosine (m6A) modification in esophageal carcinoma. Mol Biol Rep 50, 8691–8703 (2023). https://doi.org/10.1007/s11033-023-08575-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08575-2