Abstract
Background
Among the heavy metal pollution in soil, lead pollution is particularly prominent. The lead in contaminated soil will not only cause damage to plants, animals and microorganisms, but also seriously affect the progress of the entire ecosystem. Under lead stress, the abundance of DnaJ protein in plants will increase. However, little is known about the role of DnaJ in lead stress.
Methods and results
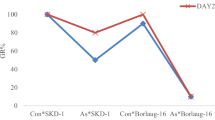
We used transgenic Arabidopsis that overexpressed DnaJ gene ZjDjB1 of Zostera japonica as material to study the role of DnaJ in the mechanism of lead induced stress response. Under lead stress, the seedlings and adult plants of transgenic ZjDjB1 Arabidopsis have higher tolerance to lead stress than wild type. Under lead stress, the content of NO and O2·− free radicals in transgenic ZjDjB1 Arabidopsis was lower than that of wild type. The negative effect of catalase in transgenic ZjDjB1 Arabidopsis under lead stress was weaker than that of wild type. The expression of ABC transporter of mitochondrion 3 (ATM3; systematic name: ABCB25) in transgenic ZjDjB1 Arabidopsis under lead stress was higher than that in wild type.
Conclusions
These results confirmed that ZjDjB1, the DnaJ gene of Z. japonica, was involved in the reaction mechanism to lead pollution, which might improve the tolerance of plants to lead stress by maintaining catalase activity and increasing the expression level of ATM3 under lead stress.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article or supplementary file. The nucleotide and deduced amino acid sequence data of ZjDjB1 were registered in GenBank (No. MN395290).
Code availability
Not applicable.
References
Damodaran D, Shetty KV, Mohan BR (2013) Effect of chelaters on bioaccumulation of cd (II), Cu (II), cr (VI), pb (II) and zn (II) in Galerina vittiformis from soil. Int Biodeterior Biodegrad 85:182–188
Tanhan P et al (2007) Uptake and accumulation of cadmium, lead and zinc by Siam weed [Chromolaena odorata (L.) King & Robinson]. Chemosphere 68(2):323–329
Estrella-Gómez N et al (2009) The Pb-hyperaccumulator aquatic fern Salvinia minima Baker, responds to Pb(2+) by increasing phytochelatins via changes in SmPCS expression and in phytochelatin synthase activity. Aquat Toxicol 91(4):320–328
Haynes D, EJohnson J (2000) Organochlorine, heavy metal and polyaromatic hydrocarbon pollutant concentrations in the great barrier reef (Australia) environment: a review. Mar Pollut Bull 41:267–278
Schlacher-Hoenlinger MA, Schlacher TA (1998) Differential accumulation patterns of heavy metals among the dominant macrophytes of a Mediterranean seagrass meadow. Chemosphere 37(8):1511–1519
Filho G et al (2004) Metal accumulation by Halodule wrightii populations. Aquat Bot 80(4):241–251
Marsh H et al (1982) Analysis of stomach contents of Dugongs from Queensland. Aust wildl res 9(1):55–67
Lin H et al (2016) Heavy metal spatial variation, bioaccumulation, and risk assessment of Zostera japonica habitat in the Yellow River estuary, China. Sci Total Environ 541:435–443
Yang X et al (2021) The bioaccumulation of Zostera japonica for five heavy metals (Zn, Cr, Cu, Pb, cd) at different growth stages in the Yellow sea and Bohai sea. Mar Environ Sci 40(6):895–902
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92(3):351–366
Chow KC, Tung WL (1998) Overexpression of dnaK/dnaJ and groEL confers freeze tolerance to Escherichia coli. Biochem Biophys Res Commun 253(2):502–505
McCarthy D, Kramer G, Hardesty B (1998) Reactivation of thermally inactivated pre-beta-lactamase by DnaK, DnaJ, and GrpE. Protein Sci 7(5):1164–1171
Chen S, Qiu G (2021) Overexpression of seagrass DnaJ gene ZjDjB1 enhances the thermotolerance of transgenic Arabidopsis thaliana. Physiol Mol Biol Plants 27(9):2043–2055
Xiang Q et al (2022) Correlation between SPAD and chlorophyll content in infected tomato leaves at different temperatures. North Hortic 18:8–15
Corpas FJ, Barroso JB (2017) Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O(2)(·−)) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide 68:103–110
Kim DY et al (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140(3):922–932
Wahsha M, Bini C, Fontana S (2012) Toxicity assessment of contaminated soils from a mining area in Northeast Italy by using lipid peroxidation assay. J Geochemi Explor 113:112–117
Besson-Bard A et al (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149(3):1302–1315
Pourrut B et al (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Phang IC et al (2011) Correlation of growth inhibition with accumulation of Pb in cell wall and changes in response to oxidative stress in Arabidopsis thaliana seedlings. Plant Growth Regul 64(1):17–25
Wang P et al (2012) Effects of Pb on the oxidative stress and antioxidant response in a pb bioaccumulator plant Vallisneria natans. Ecotoxicol Environ Saf 78:28–34
Malecka A, Jarmuszkiewicz W, Tomaszewska B (2001) Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochim Pol 48(3):687–698
Reis GS et al (2015) Molecular, biochemical and ultrastructural changes induced by Pb toxicity in seedlings of Theobroma cacao L. PLoS ONE 10(7):e0129696
Kumar A, Majeti NV (2014) Proteomic responses to lead-induced oxidative stress in Talinum triangulare Jacq. (Willd.) roots: identification of key biomarkers related to glutathione metabolisms. Environ Sci Pollut Res Int 21(14):8750–8764
Clark D et al (2000) Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact 13(12):1380–1384
Kushnir S et al (2001) A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13(1):89–100
Funding
This study was funded by the Natural Science Foundation of Guangxi Province (2020GXNSFAA297067), the Research Fund Program of Guangxi Key Lab of Mangrove Conservation and Utilization (GKLMC-22A02; GKLMC-21A01; GKLMC-20A04; GKLMC-20A01), the National Natural Science Foundation of China (32170399) and the National Science & Technology Fundamental Resources Investigation Program of China (2019FY100604). These funding bodies had no role in the design of the study, collection, analysis, and interpretation of data, or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SC designed and conducted the experiment. GQ conducted field sampling and identification. SC and GQ wrote the manuscript. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Qiu, G. Overexpression of Zostera japonica J protein gene ZjDjB1 in Arabidopsis enhanced the tolerance to lead stress. Mol Biol Rep 50, 5117–5124 (2023). https://doi.org/10.1007/s11033-023-08470-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08470-w