Abstract
Background
Osteoblast phenotypic transition in vascular smooth muscle cells (VSMCs) has been unveiled as a common cause of vascular calcification (VC). Krüppel-Associated Box (KRAB)-Associated Protein 1(KAP1) is a transcriptional corepressor that modulates various intracellular pathological processes from gene expression to DNA repair to signal transduction. However, the function and mechanism of KAP1 on the osteoblastic differentiation of VSMCs have not been evaluated yet.
Methods and results
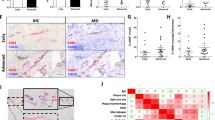
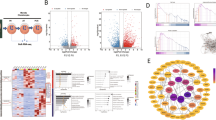
We demonstrate that the expression of KAP1 in VSMCs is significantly enhanced in vivo and in vitro calcification models. Downregulating the expression of KAP1 suppresses the osteoblast phenotypic transition of VSMCs, which is indicated by a decrease in the expression of osteoblast marker collagenase type I (COL I) and an increase in the expression of VSMC marker α-smooth muscle actin (α-SMA). Conversely, exogenous overexpression of KAP1 could promote osteoblast phenotypic transition of VSMCs. Moreover, KAP1 upregulated the expression of RUNX family transcription factor 2 (Runx2), an inducer of osteoblast that positively regulates many osteoblast-related genes, such as COL I. Evaluation of the potential mechanism demonstrated that KAP1 promoted osteoblast phenotypic transition of VSMCs by activating the extracellular regulated protein kinases (ERK) signaling pathway, which could activate Runx2. In support of this finding, KAP1-induced cell osteoblast phenotypic transition is abolished by treatment with PD0325901, a specific ERK inhibitor.
Conclusions
The present study suggested that KAP1 participated in the osteoblast differentiation of VSMCs via the ERK/Runx2 cascade and served as a potential diagnostics and therapeutics target for vascular calcification.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Change history
26 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08494-2
04 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08603-1
References
Vervloet M, Cozzolino M (2017) Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int 91:808–817
Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C (2014) Medial vascular calcification revisited: review and perspectives. Eur Heart J 35:1515–1525
Rennenberg RJMW, Kessels AGH, Schurgers LJ, van Engelshoven JMA, de Leeuw PW, Kroon AA (2009) Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 5:185–197
Cozzolino M, Ciceri P, Galassi A, Mangano M, Carugo S, Capelli I, Cianciolo G (2019) The key role of phosphate on vascular calcification. Toxins (Basel) 11:213
Smith ER (2016) Vascular calcification in uremia: new-age concepts about an old-age problem. Methods Mol Biol (Clifton, NJ) 1397:175–208
Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC (2000) Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20:8783–8792
Zhang X, Yang M, Lin L, Chen P, Ma KT, Zhou CY, Ao YF (2006) Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose–derived stem cells in vitro and in vivo. Calcif Tissue Int 79:169–178
Samatar AA, Poulikakos PI (2014) Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov 13:928–942
Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, Kolch W (2011) Raf family kinases: old dogs have learned new tricks. Genes Cancer 2:232–260
Degirmenci U, Wang M, Hu J (2020) Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells 9:198
Martinelli E, Morgillo F, Troiani T, Ciardiello F (2017) Cancer resistance to therapies against the EGFR-RAS-RAF pathway: the role of MEK. Cancer Treat Rev 53:61–69
Amann VC, Ramelyte E, Thurneysen S, Pitocco R, Bentele-Jaberg N, Goldinger SM, Dummer R, Mangana J (2017) Developments in targeted therapy in melanoma. Eur J Surg Oncol 43:581–593
Cantwell-Dorris ER, O’Leary JJ, Sheils OM (2011) BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther 10:385–394
Li B, Zhao J, Ma J-X, Li G-M, Zhang Y, Xing G-S, Liu J, Ma X-L (2018) Overexpression of DNMT1 leads to hypermethylation of H19 promoter and inhibition of Erk signaling pathway in disuse osteoporosis. Bone 111:82–91
Liu H, Li X, Qin F, Huang K (2014) Selenium suppresses oxidative-stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplasmic reticulum stress. J Biol Inorg Chem 19:375–388
Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, Rauscher FJ (1996) KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev 10:2067–2078
Matsui T, Leung D, Miyashita H, Maksakova IA, Miyachi H, Kimura H, Tachibana M, Lorincz MC, Shinkai Y (2010) Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature 464:927–931
Castro-Diaz N, Ecco G, Coluccio A, Kapopoulou A, Yazdanpanah B, Friedli M, Duc J, Jang SM, Turelli P, Trono D (2014) Evolutionally dynamic L1 regulation in embryonic stem cells. Genes Dev 28:1397–1409
Ecco G, Cassano M, Kauzlaric A, Duc J, Coluccio A, Offner S, Imbeault M, Rowe HM, Turelli P, Trono D (2016) Transposable elements and their KRAB-ZFP controllers regulate gene expression in adult tissues. Dev Cell 36:611–623
Rowe HM, Jakobsson J, Mesnard D, Rougemont J, Reynard S, Aktas T, Maillard PV, Layard-Liesching H, Verp S, Marquis J, Spitz F, Constam DB, Trono D (2010) KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463:237–240
Wolf D, Goff SP (2009) Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 458:1201–1204
Wolf D, Goff SP (2007) TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 131:46–57
Iyengar S, Ivanov AV, Jin VX, Rauscher FJ, Farnham PJ (2011) Functional analysis of KAP1 genomic recruitment. Mol Cell Biol 31:1833–1847
Bunch H, Zheng X, Burkholder A, Dillon ST, Motola S, Birrane G, Ebmeier CC, Levine S, Fargo D, Hu G, Taatjes DJ, Calderwood SK (2014) TRIM28 regulates RNA polymerase II promoter-proximal pausing and pause release. Nat Struct Mol Biol 21:876–883
Bunch H, Calderwood SK (2015) TRIM28 as a novel transcriptional elongation factor. BMC Mol Biol 16:14
McNamara RP, Reeder JE, McMillan EA, Bacon CW, McCann JL, D’Orso I (2016) KAP1 recruitment of the 7SK snRNP complex to promoters enables transcription elongation by RNA polymerase II. Mol Cell 61:39–53
D’Orso I (2016) 7SKiing on chromatin: move globally, act locally. RNA Biol 13:545–553
Lionnard L, Duc P, Brennan MS, Kueh AJ, Pal M, Guardia F, Mojsa B, Damiano M-A, Mora S, Lassot I, Ravichandran R, Cochet C, Aouacheria A, Potts PR, Herold MJ, Desagher S, Kucharczak J (2019) TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1. Cell Death Differ 26:902–917
Peng Y, Zhang M, Jiang Z, Jiang Y (2019) TRIM28 activates autophagy and promotes cell proliferation in glioblastoma. Onco Targets Ther 12:397–404
Yan T, Liang C, Fan H, Zhou W, Huang L, Qi S, Wang W, Ma P (2020) KAP1 silencing relieves OxLDL-induced vascular endothelial dysfunction by down-regulating LOX-1. Biosci Rep 40:BRS20200821
Liu H, Chen H, Deng X, Peng Y, Zeng Q, Song Z, He W, Zhang L, Xiao T, Gao G, Li B (2019) Knockdown of TRIM28 inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration. Chem Biol Interact 311:108772
Wu G-J, Pen J, Huang Y, An S, Liu Y, Yang Y, Hao Q, Guo X-X, Xu T-R (2018) KAP1 inhibits the Raf-MEK-ERK pathway to promote tumorigenesis in A549 lung cancer cells. Mol Carcinog 57:1396–1407
Bai Y, Cheng M, Jin J, Zhang H, He L, Zhou W, Zhang S, Xu J (2022) SET8, a novel regulator to ameliorate vascular calcification via activating PI3K/Akt mediated anti-apoptotic effects. Biochem Cell Biol 100:104–114
Lee JY, Park SJ, Kim DA, Lee SH, Koh J-M, Kim B-J (2020) Muscle-derived lumican stimulates bone formation via integrin α2β1 and the downstream ERK signal. Front Cell Dev Biol 8:565826
Son H-E, Jang W-G (2021) Cip2A modulates osteogenic differentiation via the ERK-Runx2 pathway in MG63 cells. BioFactors (Oxford, England) 47:658–664
Katsianou M, Papavassiliou KA, Zoi I, Gargalionis AN, Panagopoulos D, Themistocleous MS, Piperi C, Papavassiliou AG, Basdra EK (2021) Polycystin-1 modulates RUNX2 activation and osteocalcin gene expression via ERK signalling in a human craniosynostosis cell model. J Cell Mol Med 25:3216–3225
Yan Y, Liu Y, Lu R (2021) PELP1 promotes the expression of RUNX2 via the ERK pathway during the osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol 124:105078
Cammas F, Mark M, Dollé P, Dierich A, Chambon P, Losson R (2000) Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development (Cambridge, England) 127:2955–2963
Yokoe T, Toiyama Y, Okugawa Y, Tanaka K, Ohi M, Inoue Y, Mohri Y, Miki C, Kusunoki M (2010) KAP1 is associated with peritoneal carcinomatosis in gastric cancer. Ann Surg Oncol 17:821–828
Lin L-F, Li C-F, Wang W-J, Yang W-M, Wang DD-H, Chang W-C, Lee W-H, Wang J-M (2013) Loss of ZBRK1 contributes to the increase of KAP1 and promotes KAP1-mediated metastasis and invasion in cervical cancer. PLoS ONE 8:e73033
Hu M, Fu X, Cui Y, Xu S, Xu Y, Dong Q, Sun L (2015) Expression of KAP1 in epithelial ovarian cancer and its correlation with drug-resistance. Int J Clin Exp Med 8:17308–17320
Wei C, Cheng J, Zhou B, Zhu L, Khan MA, He T, Zhou S, He J, Lu X, Chen H, Zhang D, Zhao Y, Fu J (2016) Tripartite motif containing 28 (TRIM28) promotes breast cancer metastasis by stabilizing TWIST1 protein. Sci Rep 6:29822
Wang Y, Hao Y, Zhao Y, Huang Y, Lai D, Du T, Wan X, Zhu Y, Liu Z, Wang Y, Wang N, Zhang P (2020) TRIM28 and TRIM27 are required for expressions of PDGFRβ and contractile phenotypic genes by vascular smooth muscle cells. FASEB J 34:6271–6283
Blanc A, Pandey NR, Srivastava AK (2004) Distinct roles of Ca2+, calmodulin, and protein kinase C in H2O2-induced activation of ERK1/2, p38 MAPK, and protein kinase B signaling in vascular smooth muscle cells. Antioxid Redox Signal 6:353–366
Chavkin NW, Chia JJ, Crouthamel MH, Giachelli CM (2015) Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp Cell Res 333:39–48
Sedaghat S, Hoorn EJ, Ikram MA, Koop-Nieuwelink C, Kavousi M, Franco OH, van der Lugt A, Vernooij MW, Bos D (2019) Kidney function and arterial calcification in major vascular beds. J Am Heart Assoc 8:e010930
Lee SJ, Lee I-K, Jeon J-H (2020) Vascular calcification-new insights into its mechanism. Int J Mol Sci 21(8):2685
Shuai C, Guo W, Wu P, Yang W, Hu S, Xia Y, Feng P (2018) A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem Eng J 347:322–333
Funding
This work was supported by The Project of Hebei Clinical Medicine Outstanding Personnel Training (2019139), Hebei Province Medical Technology Tracking Project (G2018050), Hebei Province Key Research and Development Project (20377704D), and Hebei Province Innovation Capacity Improvement Project (20577701D).
Author information
Authors and Affiliations
Contributions
YLB and JSX conceived the study. WWB and DXZ performed the follow-up experiments and wrote the manuscript. LML performed data collections and calculations. MJC and JJJ oversaw language editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest associated with the manuscript.
Ethical approval
The experimental protocols were reviewed and approved by the Animal Care and Use Institutional Committee of The Fourth Affiliated Hospital of Hebei Medical University (No. 2020ky189).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliation is corrected as “Department of Nephrology, The Fourth Hospital of Hebei Medical University, Hebei Clinical Research Center for Chronic Kidney Disease, Hebei Key Laboratory of Vascular Calcification in Kidney Disease, Shijiazhuang P.R. China”
The original online version of this article was revised: The figures 1G, 2E and 3C have been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, W., Cheng, M., Jin, J. et al. KAP1 modulates osteogenic differentiation via the ERK/Runx2 cascade in vascular smooth muscle cells. Mol Biol Rep 50, 3217–3228 (2023). https://doi.org/10.1007/s11033-022-08225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08225-z