Abstract
Background
Today, androgen receptor (AR)-mediated signaling mechanisms in prostate cancer are intensively studied. However, the roles of other steroid hormones in prostate cancer and their effects on androgenic signaling still remain a mystery. Recent studies focused on the androgen-mediated regulation of protein quality control mechanisms such as endoplasmic reticulum-associated degradation (ERAD) and unfolded protein response (UPR) in prostate cancer cells. Present study, we investigated the action of progesterone signaling on ERAD and UPR mechanisms and analyzed the crosstalk of progesterone signaling with androgenic signal in prostate cancer cells.
Methods and results
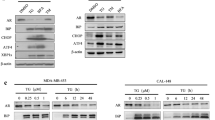
The mode of action of progesterone on ERAD, UPR and AR signaling in prostate cancer was investigated by cell culture studies using LNCaP and 22Rv1 cells. To this aim qRT-PCR, western-blotting assay, immunofluorescent microscopy, nuclear fractionation and bioinformatic analysis were used. Our results indicated that progesterone positively regulates mRNA and protein levels of ERAD components in LNCaP cells. Also, it induced the IRE⍺ and PERK branches of UPR signaling. Progesterone receptor antagonist effectively antagonized the progesterone-induced responses. We also had similar results in 22Rv1 cells. Also, we tested the effect of the pharmacologically reducing of IRE⍺ and PERK signaling on progesterone-induced ERAD. Additionally, we determined the presence of putative progesterone response elements (PREs) in the promoter regions of ERAD members by bioinformatic tool. More strikingly, we found progesterone regulates AR signaling by modulating the nuclear transactivation of AR.
Conclusion
Herein, we defined that progesterone hormone positively regulates ERAD and UPR mechanisms in prostate cancer cells and that progesterone contributes to the molecular biology of prostate cancer by regulating androgenic signaling.
Graphical abstract
Mode of Action of Progesteron on Androgen sensitive prostate cancer cells.







Similar content being viewed by others
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Sanderson JT (2006) The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94:3–21. https://doi.org/10.1093/toxsci/kfl051
Migliaccio A, Castoria G, Auricchio F (2007) Src-dependent signalling pathway regulation by sex-steroid hormones: therapeutic implications. Int J Biochem Cell Biol 39:1343–1348. https://doi.org/10.1016/j.biocel.2006.12.009
Valko-Rokytovská M, Očenáš P, Salayová A, Kostecká Z (2021) Breast cancer: targeting of steroid hormones in cancerogenesis and diagnostics. Int J Mol Sci 22:5878. https://doi.org/10.3390/ijms22115878
Capper CP, Rae JM, Auchus RJ (2016) The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm Cancer 7:149–164. https://doi.org/10.1007/s12672-016-0259-0
Chandrasekar T, Yang JC, Gao AC, Evans CP (2015) Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol 4:365–380. https://doi.org/10.3978/j.issn.2223-4683.2015.05.02
Erzurumlu Y, Ballar P (2017) Androgen mediated regulation of endoplasmic reticulum-associated degradation and its effects on prostate cancer. Sci Rep 7:40719. https://doi.org/10.1038/srep40719
Storm M, Sheng X, Arnoldussen YJ, Saatcioglu F (2016) Prostate cancer and the unfolded protein response. Oncotarget 7:54051–54066. https://doi.org/10.18632/oncotarget.9912
Lydon JP, DeMayo FJ, Funk CR et al (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278. https://doi.org/10.1101/gad.9.18.2266
Niswender GD (2002) Molecular control of luteal secretion of progesterone. Reproduction 123:333–339. https://doi.org/10.1530/rep.0.1230333
Stefanick ML (2005) Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US food and drug administration. Am J Med 118(Suppl 12B):64–73. https://doi.org/10.1016/j.amjmed.2005.10.014
Guerra-Araiza C, Gómora-Arrati P, García-Juárez M et al (2009) Role of progesterone receptor isoforms in female sexual behavior induced by progestins in rats. Neuroendocrinology 90:73–81. https://doi.org/10.1159/000224406
Brinton RD, Thompson RF, Foy MR et al (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339. https://doi.org/10.1016/j.yfrne.2008.02.001
Glass CK, Rosenfeld MG (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141. https://doi.org/10.1101/gad.14.2.121
Leonhardt SA, Edwards DP (2002) Mechanism of action of progesterone antagonists. Exp Biol Med 227:969–980. https://doi.org/10.1177/153537020222701104
Diep CH, Daniel AR, Mauro LJ et al (2015) Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol 54:31–53. https://doi.org/10.1530/JME-14-0252
Kim JJ, Chapman-Davis E (2010) Role of progesterone in endometrial cancer. Semin Reprod Med 28:81–90. https://doi.org/10.1055/s-0029-1242998
Brolin J, Skoog L, Ekman P (1992) Immunohistochemistry and biochemistry in detection of androgen, progesterone, and estrogen receptors in benign and malignant human prostatic tissue. Prostate 20:281–295. https://doi.org/10.1002/pros.2990200404
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Nabbi A, Riabowol K (2015) Rapid isolation of nuclei from cells in vitro. Cold Spring Harb Protoc 2015:db.prot083733. https://doi.org/10.1101/pdb.prot083733
Naoki T, Ikuya S, Osamu T, Keishi M (1988) RU486, a progestin antagonist, binds to progesterone receptors in a human endometrial cancer cell line and reverses the growth inhibition by progestins. J Steroid Biochem 31:161–166. https://doi.org/10.1016/0022-4731(88)90049-0
Hwang J, Qi L (2018) Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem Sci 43:593–605. https://doi.org/10.1016/j.tibs.2018.06.005
Liu L, Xu L, Zhang S et al (2018) STF-083010, an inhibitor of XBP1 splicing, attenuates acute renal failure in rats by suppressing endoplasmic reticulum stress-induced apoptosis and inflammation. Exp Anim 67:373–382. https://doi.org/10.1538/expanim.17-0131
Axten JM, Romeril SP, Shu A et al (2013) Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med Chem Lett 4:964–968. https://doi.org/10.1021/ml400228e
Sheng X, Arnoldussen YJ, Storm M et al (2015) Divergent androgen regulation of unfolded protein response pathways drives prostate cancer. EMBO Mol Med 7:788–801. https://doi.org/10.15252/emmm.201404509
Kumar VL, Majumder PK (1995) Prostate gland: structure, functions and regulation. Int Urol Nephrol 27:231–243. https://doi.org/10.1007/BF02564756
Tindall D, Lonergan P (2011) Androgen receptor signaling in prostate cancer development and progression. J Carcinog 10:20. https://doi.org/10.4103/1477-3163.83937
Jin Y, Saatcioglu F (2020) Targeting the unfolded protein response in hormone-regulated cancers. Trends Cancer 6(2):160–171. https://doi.org/10.1016/j.trecan.2019.12.001
Valle S, Sharifi N (2021) Targeting glucocorticoid metabolism in prostate cancer. Endocrinology 162(9):bqab132. https://doi.org/10.1210/endocr/bqab132
Arora VK, Schenkein E, Murali R et al (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155(6):1309–1322. https://doi.org/10.1016/j.cell.2013.11.012
Isikbay M, Otto K, Kregel S et al (2014) Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer 5(2):72–89. https://doi.org/10.1007/s12672-014-0173-2
Asher GW, Peterson AJ, Duganzich D (1989) Adrenal and ovarian sources of progesterone secretion in young female fallow deer, dama dama. J Reprod Fertil 85:667–675. https://doi.org/10.1530/jrf.0.0850667
Baker ME (2011) Origin and diversification of steroids: co-evolution of enzymes and nuclear receptors. Mol Cell Endocrinol 334:14–20. https://doi.org/10.1016/j.mce.2010.07.013
Gronemeyer H, Meyer ME, Bocquel MT et al (1991) Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol 40:271–278. https://doi.org/10.1016/0960-0760(91)90192-8
Ilhan R, Üner G, Yilmaz S et al (2022) Novel regulation mechanism of adrenal cortisol and DHEA biosynthesis via the endogen ERAD inhibitor small VCP-interacting protein. Sci Rep 12:869. https://doi.org/10.1038/s41598-022-04821-y
Guzeloglu Kayisli O, Kayisli UA, Basar M et al (2015) Progestins upregulate FKBP51 expression in human endometrial stromal cells to induce functional progesterone and glucocorticoid withdrawal: implications for contraceptive- associated abnormal uterine bleeding. PLoS ONE 10:e0137855. https://doi.org/10.1371/journal.pone.0137855
Adams CJ, Kopp MC, Larburu N et al (2019) Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci 6:11. https://doi.org/10.3389/fmolb.2019.00011
Acknowledgements
We thank Dr. Fahri Saatcioglu (Department of Biosciences, University of Oslo, Norway) for the Human prostate adenocarcinoma cell lines, LNCaP and Du145 and synthetic androgen R1881. We thank Suleyman Demirel University-Innovative Technologies Application and Research Center. Fluorescence microscopic examination was performed at Mehmet Akif Ersoy University, Veterinary Faculty, Department of Pathology, we thank Dr. Ozlem OZMEN.
Funding
This work received support from Süleyman Demirel Üniversitesi, (Grant Numbers TSG-2021-8302, TAB-2020-8253).
Author information
Authors and Affiliations
Contributions
YE initiated and directed the project, designed, and conducted the experiments, analyzed, and interpreted the results, and wrote the manuscript. HKD and DC assisted experimental studies. All correspondence and requests for materials should be addressed to YE. All authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
This study does not require any ethical permission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erzurumlu, Y., Dogan, H.K. & Catakli, D. Progesterone regulates the endoplasmic reticulum-associated degradation and Unfolded Protein Response axis by mimicking the androgenic stimulation in prostate cancer cells. Mol Biol Rep 50, 1253–1265 (2023). https://doi.org/10.1007/s11033-022-08065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08065-x