Abstract
The tumorigenic properties of prostate cancer are regulated by advanced hormonal regulation-mediated complex molecular signals. Therefore, characterizing the regulation of these signal transduction systems is crucial for understanding prostate cancer biology. Recent studies have shown that endoplasmic reticulum (ER)-localized protein quality control mechanisms, including ER-associated degradation (ERAD) and unfolded protein response (UPR) signaling contribute to prostate carcinogenesis and to the development of drug resistance. It has also been determined that these systems are tightly regulated by androgens. However, the role of estrogenic signaling in prostate cancer and its effects on protein quality control mechanisms is not fully understood. Herein, we investigated the regulatory effects of estrogens on ERAD and UPR and their impacts on prostate carcinogenesis. We found that estrogens strongly regulated the ERAD components and IRE1⍺ branch of UPR by Er⍺/β/AR axis. Besides, estrogenic signaling rigorously regulated the tumorigenicity of prostate cancer cells by promoting c-Myc expression and epithelial-mesenchymal transition (EMT). Moreover, estrogenic signal blockage significantly decreased the tumorigenic features of prostate cancer cells. Additionally, simultaneous inhibition of androgenic/estrogenic signals more efficiently inhibited tumorigenicity of prostate cancer cells, including proliferation, migration, invasion and colonial growth. Furthermore, computational-based molecular docking, molecular dynamics simulations and MMPBSA calculations supported the estrogenic stimulation of AR. Present findings suggested that ERAD components and IRE1⍺ signaling are tightly regulated by estrogen-stimulated AR and Er⍺/β. Our data suggest that treatment approaches targeting the co-inhibition of androgenic/estrogenic signals may pave the way for new treatment approaches to be developed for prostate cancer.
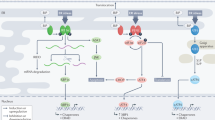
Graphical abstract
The present model of the impact of estrogens on ERAD and UPR signaling in androgen-sensitive prostate cancer cells.








Similar content being viewed by others
Data availability
The data generated in this study are available upon request from the corresponding author.
Abbreviations
- AR:
-
Androgen receptor
- ATCC:
-
American Type Tissue Culture
- BCA:
-
Bicinchoninic acid
- Bmal1:
-
Basic helix-loop-helix ARNT like 1
- BSA:
-
Bovine serum albumin
- CD82:
-
Cluster of differentiation 82
- Co-IP:
-
Co-Immunoprecipitation
- ct-FBS:
-
Charcoal-treated FBS
- E2:
-
β-estradiol
- eIF2⍺:
-
Eukaryotic Initiation Factor 2⍺
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Endoplasmic reticulum
- Er:
-
Estrogen receptor
- ERAD:
-
Endoplasmic reticulum-associated degradation
- ERQC:
-
ER quality control mechanism
- FBS:
-
Fetal Bovine Serum
- Gp78:
-
Glycoprotein 78
- GROMACS:
-
GROningen MAchine for Chemical Simulations
- GRP78:
-
Glucose-Regulated Protein 78
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl coenzyme A
- Hrd1:
-
Hydroxymethyl glutaryl-coenzyme A reductase degradation protein 1
- IP:
-
Immunoprecipitation
- IRE1⍺:
-
Inositol-requiring enzyme 1⍺
- KAI1:
-
Kangai 1
- MMPBSA:
-
Molecular mechanics Poisson-Boltzmann surface area method
- PBS:
-
Phosphate-buffered saline
- PDB:
-
Protein data bank
- PERK:
-
Protein kinase RNA-like ER kinase
- PSA:
-
Prostate specific antigen
- PTEN:
-
Phosphatase and tensin homolog
- RIPA:
-
Radioimmunoprecipitation assay
- RMSD:
-
Root mean square deviation
- RMSF:
-
Root mean square fluctuation
- RPLP0:
-
Ribosomal Protein Lateral Stalk Subunit P0
- SDS:
-
Sodium dodecyl sulfate
- TBS:
-
Tris-buffered saline
- UPR:
-
Unfolded protein response
- UV:
-
Ultraviolet
- VCP:
-
Valosin containing protein
- XBP-1:
-
X-box Binding Protein 1
References
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Adams CJ, Kopp MC, Larburu N et al (2019) Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci 6:11. https://doi.org/10.3389/fmolb.2019.00011
Aikawa K, Asano M, Ono K et al (2017) Synthesis and biological evaluation of novel selective androgen receptor modulators (SARMs) part III: discovery of 4-(5-oxopyrrolidine-1-yl) benzonitrile derivative 2f as a clinical candidate. Bioorg Med Chem 25:3330–3349. https://doi.org/10.1016/j.bmc.2017.04.018
Araki K, Nagata K (2012) Protein folding and quality control in the ER. Cold Spring Harb Perspect Biol 4:a015438. https://doi.org/10.1101/cshperspect.a015438
Ballar Kirmizibayrak P, Erbaykent-Tepedelen B, Gozen O et al (2020) Divergent modulation of proteostasis in prostate cancer. Adv Exp Med Biol 1233:117–151. https://doi.org/10.1007/978-3-030-38266-7_5
Bjelkmar P, Larsson P, Cuendet MA et al (2010) Implementation of the CHARMM Force Field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. J Chem Theory Comput 6:459–466. https://doi.org/10.1021/ct900549r
Bohl CE, Gao W, Miller DD et al (2005a) Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci U S A 102:6201–6206. https://doi.org/10.1073/pnas.0500381102
Bohl CE, Miller DD, Chen J et al (2005b) Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem 280:37747–37754. https://doi.org/10.1074/jbc.M507464200
Bouris P, Skandalis SS, Piperigkou Z et al (2015) Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol 43:42–60. https://doi.org/10.1016/j.matbio.2015.02.008
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Chen K, Han M, Tang M et al (2018) Differential Hrd1 expression and B-Cell accumulation in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res 10:698–715. https://doi.org/10.4168/aair.2018.10.6.698
Christianson JC, Ye Y (2014) Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol 21:325–335. https://doi.org/10.1038/nsmb.2793
Clarke HJ, Chambers JE, Liniker E et al (2014) Endoplasmic reticulum stress in malignancy. Cancer Cell 25:563–573. https://doi.org/10.1016/j.ccr.2014.03.015
Deshaies RJ (2014) Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol 12:94. https://doi.org/10.1186/s12915-014-0094-0
Di Zazzo E, Galasso G, Giovannelli P et al (2019) Estrogen receptors in epithelial-mesenchymal transition of prostate cancer. Cancers 11(10):1418. https://doi.org/10.3390/cancers11101418
Erzurumlu Y, Ballar P (2017) Androgen mediated regulation of endoplasmic reticulum-associated degradation and its effects on prostate cancer. Sci Rep 7:40719. https://doi.org/10.1038/srep40719
Jin Y, Saatcioglu F (2020) Targeting the unfolded protein response in hormone-regulated cancers. Trends Cancer Res 6:160–171. https://doi.org/10.1016/j.trecan.2019.12.001
Kim H, Bhattacharya A, Qi L (2015) Endoplasmic reticulum quality control in cancer: friend or foe. Semin Cancer Biol 33:25–33. https://doi.org/10.1016/j.semcancer.2015.02.003
Kim S, Chen J, Cheng T et al (2021) PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49:D1388–D1395. https://doi.org/10.1093/nar/gkaa971
Kumari R, Kumar R, Open Source Drug Discovery Consortium, et al (2014) g_mmpbsa--a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54:1951–1962. https://doi.org/10.1021/ci500020m
Liang J-S, Kim T, Fang S et al (2003) Overexpression of the tumor autocrine motility factor receptor Gp78, a ubiquitin protein ligase, results in increased ubiquitinylation and decreased secretion of apolipoprotein B100 in HepG2 cells. J Biol Chem 278:23984–23988. https://doi.org/10.1074/jbc.M302683200
Liu Y-N, Liu Y, Lee H-J et al (2008) Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol 28:7096–7108. https://doi.org/10.1128/MCB.00449-08
Liu J, Xiao M, Li J et al (2017) Activation of UPR signaling pathway is associated with the malignant progression and poor prognosis in prostate cancer. Prostate 77(3):274–281. https://doi.org/10.1002/pros.23264
Liu L, Long H, Wu Y et al (2018) HRD1-mediated PTEN degradation promotes cell proliferation and hepatocellular carcinoma progression. Cell Signal 50:90–99. https://doi.org/10.1016/j.cellsig.2018.06.011
Loh C-Y, Chai JY, Tang TF et al (2019) The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells 8(10):1118. https://doi.org/10.3390/cells8101118
Lonergan PE, Tindall DJ (2011) Androgen receptor signaling in prostate cancer development and progression. J Carcinog 10:20. https://doi.org/10.4103/1477-3163.83937
Matias PM, Donner P, Coelho R et al (2000) Structural evidence for ligand specificity in the binding domain of the human androgen receptor implications for pathogenic gene mutations. J Biol Chem 275:26164–26171. https://doi.org/10.1074/jbc.M004571200
McGrath EP, Logue SE, Mnich K et al (2018) The unfolded protein response in breast cancer. Cancers 10(10):344. https://doi.org/10.3390/cancers10100344
McInerney EM, Weis KE, Sun J et al (1998) Transcription activation by the human estrogen receptor subtype beta (ER beta) studied with ER beta and ER alpha receptor chimeras. Endocrinology 139:4513–4522. https://doi.org/10.1210/endo.139.11.6298
Migliaccio A, Castoria G, Di Domenico M et al (2000) Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417. https://doi.org/10.1093/emboj/19.20.5406
Migliaccio A, Varricchio L, De Falco A et al (2007) Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene 26(46):6619–6629. https://doi.org/10.1038/sj.onc.1210487
Muhammed MT, Kuyucuklu G, Kaynak-Onurdag F et al (2022) Synthesis, antimicrobial activity, and molecular modeling studies of some benzoxazole derivatives. Mol Divers. https://doi.org/10.2174/1570180819666220408133643
Murphy LC, Leygue E (2012) The role of estrogen receptor-β in breast cancer. Semin Reprod Med 30:5–13. https://doi.org/10.1055/s-0031-1299592
Nabbi A, Riabowol K (2015) Rapid isolation of nuclei from cells in vitro. Cold Spring Harb Protoc 2015:769–772. https://doi.org/10.1101/pdb.prot083733
Nakhla AM, Romas NA, Rosner W (1997) Estradiol activates the prostate androgen receptor and prostate-specific antigen secretion through the intermediacy of sex hormone-binding globulin. J Biol Chem 272:6838–6841. https://doi.org/10.1074/jbc.272.11.6838
Nightingale J, Chaudhary KS, Abel PD et al (2003) Ligand activation of the androgen receptor downregulates E-cadherin-mediated cell adhesion and promotes apoptosis of prostatic cancer cells. Neoplasia 5:347–361. https://doi.org/10.1016/S1476-5586(03)80028-3
Oikonomou C, Hendershot LM (2020) Disposing of misfolded ER proteins: a troubled substrate’s way out of the ER. Mol Cell Endocrinol 500:110630. https://doi.org/10.1016/j.mce.2019.110630
Pandya M, Shah S, M D, et al (2022) Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: a computational approach. Inform Med Unlocked 30:100951. https://doi.org/10.1016/j.imu.2022.100951
Pereira de Jésus-Tran K, Côté P-L, Cantin L et al (2006) Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci 15:987–999. https://doi.org/10.1110/ps.051905906
Planas-Silva MD, Waltz PK (2007) Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol 104:11–21. https://doi.org/10.1016/j.jsbmb.2006.09.039
Rawla P (2019) Epidemiology of Prostate Cancer. World J Oncol 10:63–89. https://doi.org/10.14740/wjon1191
Robinson CM, Talty A, Logue SE et al (2021) An emerging role for the unfolded protein response in pancreatic cancer. Cancers 13(2):261. https://doi.org/10.3390/cancers13020261
Roth JE, Peer CJ, Price DK et al (2014) The androgen receptor transcriptional program in castration-resistant prostate cancer: cell lines vs tissue samples. Cancer Biol Ther 15(1):16–18. https://doi.org/10.4161/cbt.27149
Saceda M, Lippman ME, Chambon P et al (1988) Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol 2:1157–1162. https://doi.org/10.1210/mend-2-12-1157
Schüttelkopf AW, van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr D Biol Crystallogr 60(8):1355–1363. https://doi.org/10.1107/s0907444904011679
Sheng X, Nenseth HZ, Qu S et al (2019) IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat Commun 10:323. https://doi.org/10.1038/s41467-018-08152-3
Sheng X, Arnoldussen YJ, Storm M, et al (2015) Divergent androgen regulation of unfolded protein response pathways drives prostate cancer. EMBO Mol Med 7:788–80. https://doi.org/10.15252/emmm.201404509
Song B-L, Sever N, DeBose-Boyd RA (2005) Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell 19:829–840. https://doi.org/10.1016/j.molcel.2005.08.009
Storm M, Sheng X, Arnoldussen YJ, et al (2016) Prostate cancer and the unfolded protein response. Oncotarget 7:54051–54066. https://doi.org/10.18632/oncotarget.9912
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Tsai YC, Mendoza A, Mariano JM et al (2007) The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med 13:1504–1509. https://doi.org/10.1038/nm1686
Veldscholte J, Ris-Stalpers C, Kuiper GG et al (1990) A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun 173:534–540. https://doi.org/10.1016/s0006-291x(05)80067-1
Walczak A, Gradzik K, Kabzinski J et al (2019) The role of the ER-induced UPR pathway and the efficacy of its inhibitors and inducers in the inhibition of tumor progression. Oxid Med Cell Longev 2019:5729710. https://doi.org/10.1155/2019/5729710
Wang F, Liu X-Q, Li H et al (2006) Structure of the ligand-binding domain (LBD) of human androgen receptor in complex with a selective modulator LGD2226. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:1067–1071. https://doi.org/10.1107/S1744309106039340
Wang Q, Li W, Zhang Y et al (2009) Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138:245–256. https://doi.org/10.1016/j.cell.2009.04.056
Wang Y, Kim S-M, Trnka MJ et al (2015) Human liver cytochrome P450 3A4 ubiquitination: molecular recognition by UBC7-gp78 autocrine motility factor receptor and UbcH5a-CHIP-Hsc70-Hsp 40 E2–E3 ubiquitin ligase complexes. J Biol Chem 290:3308–3332. https://doi.org/10.1074/jbc.M114.611525
Wang M, Ren D, Guo W et al (2016) N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. Int J Oncol 48:595–606. https://doi.org/10.3892/ijo.2015.3270
Yaşar P, Ayaz G, Muyan M (2016) Estradiol-estrogen receptor α mediates the expression of the CXXC5 gene through the estrogen response element-dependent signaling pathway. Sci Rep 6:37808. https://doi.org/10.1038/srep37808
Ying Z, Wang H, Fan H et al (2009) Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet 18:4268–4281. https://doi.org/10.1093/hmg/ddp380
Zhu M-L, Kyprianou N (2010) Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 24:769–777. https://doi.org/10.1096/fj.09-136994
Acknowledgements
We thank Suleyman Demirel University-Innovative Technologies Application and Research Center for equipmental support. We thank Dr. Ozlem Özmen (Department of Pathology, Faculty of Veterinary, Mehmet Akif Ersoy University) for allowing us to access the use of fluorescence microscope. We also thank Dr. Fahri Saatcioglu (Department of Biosciences, University of Oslo, Norway) and Dr. Mesut Muyan (Department of Biology, Faculty of Arts and Sciences, Middle East Technical University) for their generous gifts. We thank Dr. Secil Eroglu (Department of Medical Biology, Faculty of Medicine, Islam Science and Technology University) for her critical reading.
Funding
This study was supported by Suleyman Demirel University internal funds (TSG-2021-8302, TAB-2020-8253).
Author information
Authors and Affiliations
Contributions
YE initiated and directed the project, designed and conducted the experiments, analyzed and interpreted the results and wrote the manuscript. HKD, DC and EA assisted experimental studies. MTM performed computational-based molecular modeling studies. All correspondence and requests for materials should be addressed to YE. All authors have read and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
This study does not require any ethical permission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erzurumlu, Y., Dogan, H.K., Catakli, D. et al. Estrogens drive the endoplasmic reticulum-associated degradation and promote proto-oncogene c-Myc expression in prostate cancer cells by androgen receptor/estrogen receptor signaling. J. Cell Commun. Signal. 17, 793–811 (2023). https://doi.org/10.1007/s12079-022-00720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-022-00720-z