Abstract
Background
Schizophrenia is a mental illness and its pharmacological treatment consists in the administration of antipsychotics like haloperidol. However, haloperidol often causes extrapyramidal motor disorders such as tardive dyskinesia (TD). So far, there is no effective treatment against TD and alternatives for it have been sought. Isoflafones have been studied as neuroprotector and inhibitor of monoamine oxidase enzyme. Thus, the objective is to evaluate the possible protective effect of isoflavones against the induction of involuntary movements induced by haloperidol in an animal model.
Methods and results
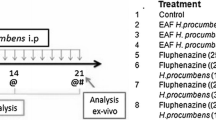
Male Wistar rats were treated with haloperidol (1 mg/kg/day) and/or isoflavones (80 mg/kg) for 28 days. Rats were submitted to behavioral evaluation to quantify vacuous chewing movements (VCM) and locomotor activity. In addition, the levels of pro-inflammatory cytokines were measured in the striatum. Haloperidol treatment reduced the locomotor activity and increased the number of VCM in rats. Co-treatment with isoflavones was able to reverse hypolocomotion and reduce the number of VCM. Besides, haloperidol caused significant increase in the proinflammatory cytokines (interleukin-1β:IL-1β, tumor necrosis factor-α: TNF-α and IL-6 and the co-treatment with isoflavones was able to reduce the levels of IL-1β and TNF-α, but not IL-6.
Conclusions
It is believed that the beneficial effect found with this alternative treatment is related to its anti-inflammatory potential and to the action on estrogen receptors (based on scientific literature findings). Finally, further studies are needed to elucidate the mechanisms of isoflavones in reducing motor disorders induced by antipsychotics.




Similar content being viewed by others
Data availability
Not applicable.
References
Ijaz S, Bolea B, Davies S, Savović J, Richards A, Sullivan S, Moran P (2018) Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry 18:275
Bhugra D (2005) The global prevalence of schizophrenia. PLoS Med 2:e151
Nardi AE, Quevedo J, Da Silva AG (2015) Esquizofrenia: teoria e clínica. Artmed Editora, Porto Alegre
Brown MJ, Sharma P, Bennett PN (2012) Clin Pharmacol. Elsevier Health Sciences, London
Amato D, Beasleyb CL, Hahn M, Vernon A (2016) Neuroadaptations to antipsychotic drugs: insights from pre-clinical and human post-mortem studies. Neurosci Biobehav 15:1–19
Witter DP, Holbert RC, Suryadevara U (2017) Pharmacotherapy for the treatment of tardive dyskinesia in schizophrenia patients. Expert Opin Pharmacother 18:965–972
Casey DE (1993) Serotonergic and dopaminergic aspects of neuroleptic-induced extrapyramidal syndromes in nonhuman primates. Psychopharmacol 112:S55–S59
Tsai G et al (1998) Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry 155:1207–1213
Glazer WM (2000) Expected incidence of tardive dyskinesia associated with atypical antipsychotics. J Clin Psychiatry 4:21–26
Liu YQ, Xin TR, Liang JJ, Wang WM, Zhang YY (2010) Memory performance, brain excitatory amino acid and acetylcholinesterase activity of chronically aluminum exposed mice in response to soy isoflavones treatment. Phytother Res 24:1451–1456
Burger M et al (2004) Effects of age on reserpine-induced orofacial dyskinesia and possible protection of diphenyl diselenide. Brain Res Bull 64:339–345
Bishnoi M, Chopra K, Kulkarni SK (2008) Activation of striatal inflammatory mediators and caspase-3 is central to haloperidol-induced orofacial dyskinesia. Eur J Pharmacol 590:241–245
Peroza LR et al (2016) Alteration of cytokines levels in the striatum of rats: possible participation in vacuous chewing movements induced by antipsycotics. Neurochem Res 41:2481–2489
Lohr JB (1991) Oxygen radicals and neuropsychiatric illness. Some speculations. Arch Gen Psychiatry 48:1097–1106
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87:10–20
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47
Peroza LR et al (2013) Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro. Neurochem Res 38:789–796
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76
Dixon RA (2004) Phytoestrogens. Annu Rev Plant Biol 55:225–261
Wei H, Wei L, Frenkel K, Bowen R, Barnes S (1993) Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer 20:1–12
Da Silva Schmitz I et al (2019) Isoflavones prevent oxidative stress and inhibit the activity of the enzyme monoamine oxidase in vitro. Mol Biol Rep 46:2285–2292
Ding BJ et al (2011) Soybean isoflavone alleviates β-amyloid 1-42 induced inflammatory response to improve learning and memory ability by down regulation of toll-like receptor 4 expression and nuclear factor-κB activity in rats. Int J Dev Neurosci 29:537–542
Fachinetto R et al (2007) Valeriana officinalis does not alter the orofacial dyskinesia induced by haloperidol in rats: role of dopamine transporter. Prog Neuropsychopharmacol Biol Psychiatry 31:1478–1486
Broadhurst PL (1960) The place of animal psychology in the development of psychosomatic research. In: European conference on psychosom res. Karger Publishers 63:69
Stahl SM (2014) Psicofarmacologia: bases neurocientíficas e aplicações práticas. Guanabara Koogan, Rio de Janeiro
Moreno RA et al (2004) Anticonvulsivantes e antipsicóticos no tratamento de transtorno bipolar. Psiquiatr 26:37–43
Reynoso SF, Davalos RM, Garcia RR, Agraz FP (2012) Estigma y apego al tratamiento psiquiatrico en los trastornos mentales severos y persistentes. Rev Latinoam Psiquiatría 11:82
Valencia M, Diaz A, Juarez F (2012) Integration of pharmacological and psychosocial treatment for schizophrenia in Mexico: the case of a developing country proposal. In: Badria F (ed) Pharmacotherapy. Intech, Europe, Croatia, pp 41–68
Ocaña-Zurita MC, Juárez-Rojop IE, Genis A et al (2016) Potential drug-drug interaction in Mexican patients with schizophrenia. Int J Psychiatry Clin Pract 20:249–253. https://doi.org/10.1080/13651501.2016.1213854
Egan MF, Hurd Y, Ferguson J, Bachus SE, Hamid EH, Hyde TM (1996) Pharmacological and neurochemical differences between acute and tardive vacuous chewing movements induced by haloperidol. Psychopharmacol 127:337–345. https://doi.org/10.1007/s002130050095
Kawakami Y et al (2004) Regulative actions of dietary soy isoflavone on biological antioxidative system and lipid metabolism in rats. J Agric Food Chem 52:1764–1768
Kelley AE, Bakshi VP, Delfs JM, Lang CG (1989) Cholinergic stimulation of the ventrolateral striatum elicits mouth movements in rats: pharmacological and regional specificity. Psychopharmacol (Berl) 99:542–549
Salamone JD, Ishiwari K, Betz AJ, Farrar AM, Mingote SM, Font L, Hockemeyer J, Muller CE, Correa M (2008) Dopamine/adenosine interactions related to locomotion and tremor in animal models: possible relevance to parkinsonism. Parkinsonism Relat Disord 2:130–134
Long T, Yao JK, Li J et al (2019) Estradiol and selective estrogen receptor agonists differentially affect brain monoamines and amino acids levels in transitional and surgical menopausal rat models. Mol Cell Endocrinol 496:110533. https://doi.org/10.1016/j.mce.2019.110533
Morito K et al (2001) Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 24:351–356
Cederroth CR, Nef S (2009) Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol 304:30–42
Essawy AE, Abdou HM, Ibrahim HM, Bouthahab NM (2019) Soybean isoflavone ameliorates cognitive impairment, neuroinflammation, and amyloid β accumulation in a rat model of Alzheimer’s disease. Environ Sci Pollut Res Int 26:26060–26070. https://doi.org/10.1007/s11356-019-05862-z
Meza DLM, Mercado CR, Barraza CA (2015) The effects of soybean isoflavones over the boné health of adult and children. Revista Salud Uninorte 31:138–152
Bains M, Roberts JL (2016) Estrogen protects against dopamine neuron toxicity in primary mesencephalic cultures through an indirect P13K/Akt mediated astrocyte pathway. Neurosci Lett 610:79–85. . doi:10.1016/j.neulet.2015.10.054
Acknowledgements
We acknowledge Universidade Franciscana for Izaviany Schmitz da Silva’s scientific initiation grant (PIBIC-UFN). Gilson Pires Dorneles is supported by a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD/CAPES). Carina Rodrigues Boeck and Pedro Roosevelt Torres Romao are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the productivity scholarship granted.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
NFM and LRP—development of experiments, data analysis, and writing manuscript. ISS, VBL, LFS and CRB—animal behavioral analysis and data analysis. GPD and PRTR—cytokine analysis and data analysis. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not hold any conflict of interest.
Ethical approval
The experimental protocol was approved by the Comissão de Ética no Uso de Animais (CEUA) of Universidade Franciscana (UFN) under the number 05/2017.
Consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mezzomo, N.F., da Silva Schmitz, I., de Lima, V.B. et al. Reversal of haloperidol-induced orofacial dyskinesia and neuroinflammation by isoflavones. Mol Biol Rep 49, 1917–1923 (2022). https://doi.org/10.1007/s11033-021-07003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07003-7