Abstract
Background
DNA methylation is one of the most important epigenetic event that regulates gene expression. In addition to DNA methylation, transgene copy number may induce gene silencing. Therefore, the study of these cases is useful for understanding of gene silencing regulation.
Methods and results
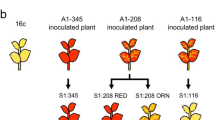
In this study, the methylation pattern of 35S promoter was investigated in the second generation of MAP30 transgenic tobacco lines. Therefore, the genomic DNA melting curve changes were investigated before and after bisulfite treatment by real time PCR. To determine the exact position of methylation, the samples were sequenced after bisulfite treatment. Observation of decrease in DNA melting curve of expressing line in comparison with silenced line confirmed the presence of DNA methylation in silenced line. In order to induce the MAP30 expression, the silenced line was treated using different concentrations of Azacytidine and green tea extracts. The results showed that all concentrations of green tea extracts for 6 days and the concentrations of 3 and 10 μM Azacytidine for 10 and 3 days could induce the expression of MAP30 in silenced line respectively. Finally, the transgene copy number was estimated using real time PCR, as silenced line contained more than two copies while the lines expressing MAP30 contained only one or two copies.
Conclusions
Finally, we found that the presence of DNA methylation and also multiple gene copy numbers in silenced line have been led to gene silencing. Moreover, the effect of green tea extract on DNA methylation showed incredible results for the first time.




Similar content being viewed by others
Notes
EF is a housekeeping gene that because of its stable expression in varying conditions was set as reference gene.
virG is a bacterial gene and used for identification of bacterial infection of transgenic plants.
Axi1 gene, involved in auxin action, have a constant number per genome with a linear ΔCt in tobacco plants.
References
Atkinson RG, Bieleski LRF, Gleave AP, Janssen BJ, Morris BAM (1998) Post-transcriptional silencing of chalcone synthase in Petunia using a geminivirus-based episomal vector. Plant J 15(5):593–604. https://doi.org/10.1046/j.1365-313X.1998.00211.x
Emani C, Sunilkumar G, Rathore KS (2002) Transgene silencing and reactivation in sorghum. Plant Sci 162(2):181–192. https://doi.org/10.1016/S0168-9452(01)00559-3
Fan J, Liu X, Xu SX, Xu Q, Guo WW (2011) T-DNA direct repeat and 35S promoter methylation affect transgene expression but do not cause silencing in transgenic sweet orange. Plant Cell, Tissue Organ Cult 107(2):225–232. https://doi.org/10.1007/s11240-011-9973-z
Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS (2003) Tea Polyphenol (-)-Epigallocatechin-3-Gallate Inhibits DNA Methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Can Res 63(22):7563–7570
Fojtová M, Bleys A, Bedřichová J, Van Houdt H, Křížová K, Depicker A, Kovařík A (2006) The trans-silencing capacity of invertedly repeated transgenes depends on their epigenetic state in tobacco. Nucleic Acids Res 34(8):2280–2293. https://doi.org/10.1093/nar/gkl180
Gallego-Bartolomé J (2020) DNA methylation in plants: mechanisms and tools for targeted manipulation. New Phytol 227(1):38–44. https://doi.org/10.1111/nph.16529
Gao W, Li S, Li Z, Huang Y, Deng C, Lu L (2014) Detection of genome DNA methylation change in spinach induced by 5-azaC. Mol Cell Probes 28(4):163–166. https://doi.org/10.1016/j.mcp.2014.02.002
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15(6):851–864. https://doi.org/10.1007/BF00039425
Hobbs SLA, Warkentin TD, DeLong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21(1):17–26. https://doi.org/10.1007/BF00039614
Ingham DJ, Beer S, Money S, Hansen G (2001) Quantitative real-time PCR assay for determining transgene copy number in transformed plants. Biotechniques 31(1):132–140. https://doi.org/10.2144/01311rr04
John MC, Amasino RM (1989) Extensive changes in DNA methylation patterns accompany activation of a silent T-DNA ipt gene in Agrobacterium tumefaciens-transformed plant cells. Mol Cell Biol 9(10):4298–4303. https://doi.org/10.1128/mcb.9.10.4298
Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: Comparison of sense vs antisense constructs and single-copy vs complex T-DNA sequences. Plant Mol Biol 31(5):957–973. https://doi.org/10.1007/BF00040715
Ju Z, Wang L, Cao D, Zuo J, Zhu H, Fu D, Luo Y, Zhu B (2016) A viral satellite DNA vector-induced transcriptional gene silencing via DNA methylation of gene promoter in Nicotiana benthamiana. Virus Res 223:99–107. https://doi.org/10.1016/j.virusres.2016.07.005
Khatodia S, Kharb P, Batra P, Chowdhury VK (2014) Real time PCR based detection of transgene copy number in transgenic chickpea lines expressing Cry1Aa3 and Cry1Ac. Int J Pure App Biosci 2(4):100–105
Kiselev KV, Dubrovina AS, Tyunin AP (2015) The methylation status of plant genomic DNA influences PCR efficiency. J Plant Physiol 175:59–67. https://doi.org/10.1016/j.jplph.2014.10.017
Kovavik A, Koukalova B, Holy A, Bezdek M (1994) Sequence-specific hypomethylation of the tobacco genome induced with dihydroxypropyladenine, ethionine and 5-azacytidine. FEBS Lett 353(3):309–311. https://doi.org/10.1016/0014-5793(94)01048-X
Ku, M. S. B., Agarie, S., Nomura, M., Fukayama, H., Tsuchida, H., Ono, K., Hirose, S., Toki, S., Miyao, M., & Matsuoka, M. (1999). High-level expression of maize phospho enol pyruvate carboxylase in transgenic rice plants. 17(January).
Li Z, Hansen JL, Liu Y, Zemetra RS, Berger PH (2004) Using real-time PCR to determine transgene copy number in wheat. Plant Mol Biol Report 22(2):179–188. https://doi.org/10.1007/BF02772725
Marenkova TV, Loginova DB, Deineko EV (2012) Mosaic patterns of transgene expression in plants. Russ J Genet 48(3):249–260. https://doi.org/10.1134/S1022795412030088
Moghadam A, Niazi A, Afsharifar A, Taghavi SM (2016) Expression of a recombinant anti-HIV and anti-tumor protein, MAP30, in nicotiana tobacum hairy roots: A pH-stable and thermophilic antimicrobial protein. PLoS ONE 11(7):1–27. https://doi.org/10.1371/journal.pone.0159653
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Okumura A, Shimada A, Yamasaki S, Horino T, Iwata Y, Koizumi N, Nishihara M, Mishiba K, ichiro. (2016) CaMV-35S promoter sequence-specific DNA methylation in lettuce. Plant Cell Rep 35(1):43–51. https://doi.org/10.1007/s00299-015-1865-y
Pan X, Niu G, Liu H (2003) Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process 42(2):129–133. https://doi.org/10.1016/S0255-2701(02)00037-5
Paszkowski J, Whitham SA (2001) Gene silencing and DNA methylation processes. Curr Opin Plant Biol 4(2):123–129. https://doi.org/10.1016/S1369-5266(00)00147-3
Que Q, Wang HY, English JJ, Jorgensen RA (1997) The frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene promoter strength and are reduced by premature nonsense codons in the transgene coding sequence. Plant Cell 9(8):1357–1368. https://doi.org/10.1105/tpc.9.8.1357
Sano H, Kamada I, Youssefian S, Katsumi M, Wabiko H (1990) A single treatment of rice seedlings with 5-azacytidine induces heritable dwarfism and undermethylation of genomic DNA. MGG Molecular & General Genetics 220(3):441–447. https://doi.org/10.1007/BF00391751
Slogteren, G. M. S. Van, Schilperoort, R. A., & Group, M. (1984). Activated By Grafting and By 5-Azaeytidine Treatment. 336, 333–336.
Costa, J., Lopes, S., Barros, B., Carneiro, A., & de Sousa, S. M. (2017). Determination of tobacco transgene copy number by real-time quantitative PCR. Simpósio Brasileiro de Genética Molecular de Plantas, 6.
Subr Z, Novakova S, Drahovska H (2006) Detection of transgene copy number by analysis of the T1 generation of tobacco plants with introduced P3 gene of potato virus A. Acta Virol 50(2):135–138
Tang W, Newton RJ, Weidner DA (2007) Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J Exp Bot 58(3):545–554. https://doi.org/10.1093/jxb/erl228
Weinhold A, Kallenbach M, Baldwin IT (2013) Progressive 35S promoter methylation increases rapidly during vegetative development in transgenic Nicotiana attenuata plants. BMC Plant Biol. https://doi.org/10.1186/1471-2229-13-99
Yuan JS, Burris J, Stewart NR, Mentewab A, Neal CN (2007) Statistical tools for transgene copy number estimation based on real-time PCR. BMC Bioinformatics 8(SUPPL. 7):1–12. https://doi.org/10.1186/1471-2105-8-S7-S6
Zwergel C, Valente S, Mai A (2015) DNA methyltransferases inhibitors from natural sources. Curr Top Med Chem 16(7):680–696. https://doi.org/10.2174/1568026615666150825141505
Acknowledgements
The authors would like to thank the Institute of Biotechnology and Department of Plant protection for supporting this research in the College of Agriculture (Shiraz University).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravanrouy, F., Niazi, A., Moghadam, A. et al. MAP30 transgenic tobacco lines: from silencing to inducing. Mol Biol Rep 48, 6719–6728 (2021). https://doi.org/10.1007/s11033-021-06662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06662-w