Abstract
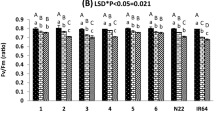
Drought stress is one of the main problems for the rice crop, as it reduces the production and productivity of the grain yield significantly. In Egypt, many restrictions were made on the cultivation of rice due to its high-water demand. Producing promising drought-tolerant rice cultivars combined with high yielding is one of the main targets for rice breeders. A set of 22 highly diverse rice genotypes were evaluated under normal and drought conditions. Morphological, physiological, and yield traits were recorded on each genotype. Drought susceptibility index (DSI) was estimated for six yield traits to identify the most drought-tolerant rice genotypes. A high genetic variation was found among genotypes tested in the experiment. Under normal conditions, the highest phenotypic correlation was found between grain yield (GY) and sterility percentage (SP) (− 0.73**), while it was among GY and chlorophyll content (CC) (0.82**) under drought conditions. To identify quantitative trait loci (QTL) controlling yielding traits under drought and normal, a single marker analysis was performed between all yield traits under both conditions and a set of 106 simple sequence repeat (SSR) marker alleles. The genetic association analysis revealed 14 and 17 QTL under drought and normal conditions, respectively. The most drought-tolerant genotypes were selected based on phenotypic traits, the number of QTL in each selected genotype, and the level of genetic diversity existed among the genotypes. As a result, five genotypes (Giza 178, IET1444, GZ1368-S-5-4, Nahda, Giza 14) were identified as the most promising drought-tolerant rice genotypes. Eight QTL controlling drought tolerance were identified in Giza 178, Nahda, and GZ1368-S-5-4, while four QTL were found in IET1444. The number of different QTLs were estimated among the five selected genotypes. Giza 178 and GZ1368-S-5-4 shared the same QTLs. Seven different QTLs were found among Nahda, IET1444, GZ1368-S-5-4, and Giza 14. Combining information from phenotypic traits, genetic diversity analysis, and QTL analysis was very useful in identifying the true drought-tolerant rice genotypes that can be used for crossing in the future breeding program.





Similar content being viewed by others
Abbreviations
- QTL:
-
Quantitative trait loci
- SMA:
-
Single marker analysis
- DTF:
-
Days to flowering
- Ph:
-
Plant height
- CC:
-
Chlorophyll content
- LR:
-
Leaf rolling
- FLA:
-
Flag leaf area
- PL:
-
Panicle length
- NOP:
-
Number of panicles
- HGW:
-
100-Grain weight
- SP:
-
Sterility percentage
- GY:
-
Grain yield
- G1:
-
Subpopulation 1
- G1:
-
Subpopulation 2
References
IRRI (2004) International Rice Research Institute. Annual Technical Report. Los Banous
AbdAllah AA (2010) Development of some high yielding rice lines tolerant to drought stress conditions. J Med Plants Res 4:528–535
AbdAllah AA, Abdel-Hafez AG, El Degwy IS, Ghazy MI (2013) Root characters studied in relation to drought and heat tolerance in some rice genotypes. The 8th Plant Breeding International Conference 14–15 May 2013. Egypt. J Plant Breed 17(2):131–146
Kusaka M, Ohta M, Fujimura T (2005) Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiol Plant 125:474–489. https://doi.org/10.1111/j.1399-3054.2005.00578.x
Shao H, Chu L, Lu Z, Kang C (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Sallam A, Mourad AMI, Hussain W, Stephen Baenziger P (2018) Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 214:169. https://doi.org/10.1007/s10681-018-2245-9
Lazar MD, Salisbury CD, Worrall WD (1995) Variation in drought susceptibility among closely related wheat lines. Field Crop Res 41:147–153. https://doi.org/10.1016/0378-4290(95)00015-I
Kadam S, Singh K, Shukla S et al (2012) Genomic associations for drought tolerance on the short arm of wheat chromosome 4B. Funct Integr Genom 12:447–464. https://doi.org/10.1007/s10142-012-0276-1
Salem KFM, Sallam A (2016) Analysis of population structure and genetic diversity of Egyptian and exotic rice (Oryza sativa L.) genotypes. C R Biol 339:1–9. https://doi.org/10.1016/j.crvi.2015.11.003
Sukumaran S, Reynolds MP, Sansaloni C (2018) Genome-wide association analyses identify QTL hotspots for yield and component traits in durum wheat grown under yield potential, drought, and heat stress environments. Front Plant Sci 9:81. https://doi.org/10.3389/fpls.2018.00081
Sandhu N, Singh A, Dixit S et al (2014) Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genet 15:63. https://doi.org/10.1186/1471-2156-15-63
Hussain W, Guttieri MJ, Belamkar V et al (2018) Registration of a bread wheat recombinant inbred line mapping population derived from a cross between ‘Harry’ and ‘Wesley.’ J Plant Regist. https://doi.org/10.3198/jpr2017.11.0085crmp
Eltaher SS, Sallam A, Belamkar V et al (2018) Genetic diversity and population structure of F3:6 Nebraska winter wheat genotypes using genotyping-by-sequencing. Front Genet 9:76. https://doi.org/10.3389/FGENE.2018.00076
Mourad AMI, Sallam A, Belamkar V et al (2018) Genome-wide association study for identification and validation of novel SNP markers for Sr6 stem rust resistance gene in bread wheat. Front Plant Sci 9:380. https://doi.org/10.3389/fpls.2018.00380
Ovenden B, Milgate A, Wade LJ et al (2017) Genome-wide associations for water-soluble carbohydrate concentration and relative maturity in wheat using SNP and DArT marker arrays. G3 7:2821–2830. https://doi.org/10.1534/g3.117.039842
Qaseem MF, Qureshi R, Muqaddasi QH et al (2018) Genome-wide association mapping in bread wheat subjected to independent and combined high temperature and drought stress. PLoS One 13:e0199121. https://doi.org/10.1371/journal.pone.0199121
Ahmed M, Kamran A, Asif M et al (2013) Silicon priming: a potential source to impart abiotic stress tolerance in wheat : a review. Aust J Crop Sci 7:484–491
Tabkhkar N, Rabiei B, Samizadeh Lahiji H, Hosseini Chaleshtori M (2018) Genetic variation and association analysis of the SSR markers linked to the major drought-yield QTLs of rice. Biochem Genet 56:356–374. https://doi.org/10.1007/s10528-018-9849-6
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86. https://doi.org/10.1186/s12870-016-0771-y
Barik SR, Pandit E, Pradhan SK et al (2019) Genetic mapping of morpho-physiological traits involved during reproductive stage drought tolerance in rice. PLoS One 14:e0214979. https://doi.org/10.1371/journal.pone.0214979
de Datta SK, Malabuyoc JA, Aragon EL (1988) A field screening technique for evaluating rice germplasm for drought tolerance during the vegetative stage. Field Crop Res 19:123–134. https://doi.org/10.1016/0378-4290(88)90050-0
Utz HF (1991) A computer program for statistical analysis of plant breeding experiments. Version 2N. Institute of Plant Breeding, Seed Science and Population Genetics. University of Hohenheim
McCouch SR, Kochert G, Yu ZH et al (1988) Molecular mapping of rice chromosomes. Theor Appl Genet 76:815–829. https://doi.org/10.1007/BF00273666
Akram HM, Ali A, Sattar A et al (2013) Impact of water deficit stress on various physiological and agronomic traits of three basmati rice (Oryza sativa L.) cultivars. J Anim Plant Sci 23(5):1415–1423
Mukamuhirwa A (2015) Drought responses of rice (Oryza sativa) under various drought severity levels and durations in biotron and field. Introductory paper at the Faculty of Landscape Architecture, Horticulture and Crop Production Science 2015:1
Sallam A, Ghanbari M, Martsch R (2017) Genetic analysis of winter hardiness and effect of sowing date on yield traits in winter faba bean. Sci Hortic (Amst) 224:296–301. https://doi.org/10.1016/j.scienta.2017.04.015
Hazman M, Brown KM (2018) Progressive drought alters architectural and anatomical traits of rice roots. Rice 11:62. https://doi.org/10.1186/s12284-018-0252-z
Elshafei A, Barakat M, Milad S et al (2019) Regeneration of rice somaclons tolerant to high level of abscisic acid and their characterization via RAPD markers. Bull Natl Res Cent 43:107. https://doi.org/10.1186/s42269-019-0154-2
Casartelli A, Riewe D, Hubberten HM et al (2018) Exploring traditional aus-type rice for metabolites conferring drought tolerance. Rice 11:9. https://doi.org/10.1186/s12284-017-0189-7
Mohamed A, Sedeek S, Galal A, Alsakka M (2019) Effect of water deficiency as abiotic stress on the reproductive and ripening stage of rice genotypes. Int J Plant Sci Agric 2:13–19
Sallam A, Dhanapal AP, Liu S (2016) Association mapping of winter hardiness and yield traits in faba bean (Vicia faba L.). Crop Pasture Sci 67:55. https://doi.org/10.1071/CP15200
Ramchander S, Robin S, Raveendran M (2016) Mapping QTLs for physiological traits associated with drought tolerance in rice (Oryza sativa L.). J Investig Genom. https://doi.org/10.15406/jig.2016.03.00052
Amar S, Ecke W, Becker HC, Möllers C (2008) QTL for phytosterol and sinapate ester content in Brassica napus L. collocate with the two erucic acid genes. Theor Appl Genet 116:1051–1061. https://doi.org/10.1007/s00122-008-0734-2
Basunanda P, Radoev M, Ecke W et al (2010) Comparative mapping of quantitative trait loci involved in heterosis for seedling and yield traits in oilseed rape (Brassica napus L.). Theor Appl Genet 120:271–281. https://doi.org/10.1007/s00122-009-1133-z
Liu G, Mei H, Liu H et al (2010) Sensitivities of rice grain yield and other panicle characters to late-stage drought stress revealed by phenotypic correlation and QTL analysis. Mol Breed 25:603–613. https://doi.org/10.1007/s11032-009-9356-x
Yue B, Xiong L, Xue W et al (2005) Genetic analysis for drought resistance of rice at reproductive stage in field with different types of soil. Theor Appl Genet 111:1127–1136. https://doi.org/10.1007/s00122-005-0040-1
Wang J, Sun J, Li C et al (2016) Genetic dissection of the developmental behavior of plant height in rice under different water supply conditions. J Integr Agric 15:2688–2702. https://doi.org/10.1016/S2095-3119(16)61427-2
Ikeda H, Kamoshita A, Manabe T (2006) Genetic analysis of rooting ability of transplanted rice (Oryza sativa L.) under different water conditions. J Exp Bot 58:309–318. https://doi.org/10.1093/jxb/erl162
Xu JL, Lafitte HR, Gao YM et al (2005) QTLs for drought escape and tolerance identified in a set of random introgression lines of rice. Theor Appl Genet 111:1642–1650. https://doi.org/10.1007/s00122-005-0099-8
Shamsudin NAA, Swamy BPM, Ratnam W et al (2016) Pyramiding of drought yield QTLs into a high quality Malaysian rice cultivar MRQ74 improves yield under reproductive stage drought. Rice (NY) 9:21. https://doi.org/10.1186/s12284-016-0093-6
Venuprasad R, Bool ME, Quiatchon L et al (2012) A large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol Breed 30:535–547. https://doi.org/10.1007/s11032-011-9642-2
Yadav S, Sandhu N, Majumder RR et al (2019) Epistatic interactions of major effect drought QTLs with genetic background loci determine grain yield of rice under drought stress. Sci Rep 9:2616. https://doi.org/10.1038/s41598-019-39084-7
Onde RD, Umar JK, Ouda GG et al (2019) Assessment of genetic diversity of drought tolerant and susceptible rice genotypes using microsatellite markers. Rice Sci. https://doi.org/10.1016/j.rsci.2019.01.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghazy, M.I., Salem, K.F.M. & Sallam, A. Utilization of genetic diversity and marker-trait to improve drought tolerance in rice (Oryza sativa L.). Mol Biol Rep 48, 157–170 (2021). https://doi.org/10.1007/s11033-020-06029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06029-7