Abstract
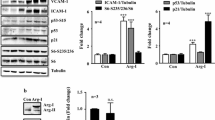
Oxidative DNA damage contributes to replicative senescence. We explored the mechanism by which angiotensin II (Ang II) induces senescence in human vascular endothelial cells (HUVECs). Following weeklong incubation with Ang II, cell senescence, apoptosis, reactive oxygen species (ROS) content and mitochondrial membrane potential (MMP) were measured by β-galactosidase, annexin V/propidium iodide, DCFH-DA and rhodamine 123 staining, respectively. The protein levels of telomerase reverse transcriptase (TERT), UCP2, Akt, phosphor (p)-Akt, c-myc, and p53 were assessed by immunoblot. LY294002 was applied to inhibit PI3K/Akt signaling. Ang II induced HUVEC senescence and apoptosis, and increased ROS content and depolarization of MMP in a dose-dependent manner. Ang II further elevated protein levels of TERT from 0.006 ± 0.041 at baseline, to 0.480 ± 00.031 in the presence of 10 µM Ang II, UCP2 from 0.297 ± 0.051 to 2.512 ± 0.024, p-Akt from 0.012 ± 0.024 to 0.874 ± 0.015, c-myc from 0.521 ± 0.015 to 1.064 ± 0.025, and p53 from 0.035 ± 0.047 to 1.195 ± 0.029 (all P < 0.01, vs. baseline). LY294002 pre-treatment significantly alleviated Ang II-induced HUVEC senescence, and partly reversed the elevation of TERT, UCP2, p-Akt, c-myc and p53 protein levels. PI3K/Akt/UCP2 signaling may be involved in cell senescence and apoptosis induced by Ang II in HUVECs.





Similar content being viewed by others
References
Cichowski K, Hahn WC (2008) Unexpected pieces to the senescence puzzle. Cell 133:958–961
Burhans WC, Weinberger M (2007) DNA replication stress, genome instability and aging. Nucleic Acids Res 35:7545–7556
Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP et al (2009) Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J 23:783–794
Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117:2417–2426
Voghel G, Thorin-Trescases N, Mamarbachi AM, Villeneuve L, Mallette FA, Ferbeyre G et al (2010) Endogenous oxidative stress prevents telomerase-dependent immortalization of human endothelial cells. Mech Ageing Dev 131:354–363
Wilson SK (1990) Role of oxygen-derived free radicals in acute angiotensin II–induced hypertensive vascular disease in the rat. Circ Res 66:722–734
Shan H, Bai X, Chen X (2008) Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem Funct 26:459–466
Shan HY, Bai XJ, Chen XM (2008) Apoptosis is involved in the senescence of endothelial cells induced by angiotensin II. Cell Biol Int 32:264–270
Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK (1996) p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271:23317–23321
Wang J, Sun P, Bao Y, Dou B, Song D, Li Y (2012) Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol In Vitro 26:32–41
Paravicini TM, Touyz RM (2006) Redox signaling in hypertension. Cardiovasc Res 71:247–258
Denu JM, Tanner KG (1998) Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37:5633–5642
Garinis GA, Schumacher B (2009) Transcription-blocking DNA damage in aging and longevity. Cell Cycle 8:2134–2135
Bitar MS, Ayed AK, Abdel-Halim SM, Isenovic ER, Al-Mulla F (2010) Inflammation and apoptosis in aortic tissues of aged type II diabetes: amelioration with alpha-lipoic acid through phosphatidylinositol 3-kinase/Akt- dependent mechanism. Life Sci 86:844–853
Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY et al (2005) High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol 25:539–545
Chen T, Ding G, Jin Z, Wagner MB, Yuan Z (2012) Insulin ameliorates miR-1-induced injury in H9c2 cells under oxidative stress via Akt activation. Mol Cell Biochem 369:167–174
Hart JR, Vogt PK (2011) Phosphorylation of AKT: a mutational analysis. Oncotarget 2:467–476
Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H et al (2013) Klotho suppresses tumor progression via inhibiting PI3K/Akt/GSK3beta/Snail signaling in renal cell carcinoma. Cancer Sci 104:663–671
Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71
Xin M, Deng X (2005) Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem 280:10781–10789
Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV (2010) The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2:344–352
Demidenko ZN, Shtutman M, Blagosklonny MV (2009) Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle 8:1896–1900
Chung J, Khadka P, Chung IK (2012) Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci 125:2684–2697
Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K et al (2004) TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochem Biophys Res Commun 316:528–532
Bellon M, Nicot C (2008) Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood 112:2946–2955
Park JY, Park KG, Kim HJ, Kang HG, Ahn JD, Kim HS et al (2005) The effects of the overexpression of recombinant uncoupling protein 2 on proliferation, migration and plasminogen activator inhibitor 1 expression in human vascular smooth muscle cells. Diabetologia 48:1022–1028
Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei X et al (2013) Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem 32:1167–1177
Rose G, Crocco P, De Rango F, Montesanto A, Passarino G (2011) Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS One 6:e29650
Xiao F, Zheng X, Cui M, Shi G, Chen X, Li R et al (2011) Telomere dysfunction-related serological markers are associated with type 2 diabetes. Diabetes Care 34:2273–2278
Kuhla A, Trieglaff C, Vollmar B (2011) Role of age and uncoupling protein-2 in oxidative stress, RAGE/AGE interaction and inflammatory liver injury. Exp Gerontol 46:868–876
Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K et al (2010) Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209:42–50
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88:323–331
Feng Z, Levine AJ (2010) The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 20:427–434
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35:505–513
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA et al (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99
Derdak Z, Fulop P, Sabo E, Tavares R, Berthiaume EP, Resnick MB et al (2006) Enhanced colon tumor induction in uncoupling protein-2 deficient mice is associated with NF-kappaB activation and oxidative stress. Carcinogenesis 27:956–961
Dietrich MO, Horvath TL (2010) The role of mitochondrial uncoupling proteins in lifespan. Pflugers Arch 459:269–275
Brand MD (2000) Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35:811–820
Holzenberger M, Dupont J, Ducos B et al (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187
Speakman JR, Talbot DA, Selman C et al (2004) Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3:87–95
Fridell YW, Sánchez-Blanco A, Silvia BA, Helfand SL (2005) Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 1:145–152
Conti B, Sanchez-Alavez M, Winsky-Sommerer R et al (2006) Transgenic mice with a reduced core body temperature have an increased life span. Science 314:825–828
Pelengaris S, Khan M, Evan G (2002) c-MYC: more than just a matter of life and death. Nat Rev Cancer 2:764–776
Yeh ES, Belka GK, Vernon AE, Chen CC, Jung JJ, Chodosh LA (2013) Hunk negatively regulates c-myc to promote Akt-mediated cell survival and mammary tumorigenesis induced by loss of Pten. Proc Natl Acad Sci U S A 110:6103–6108
Horikawa I, Cable PL, Mazur SJ, Appella E, Afshari CA, Barrett JC (2002) Downstream E-box-mediated regulation of the human telomerase reverse transcriptase (hTERT) gene transcription: evidence for an endogenous mechanism of transcriptional repression. Mol Biol Cell 13:2585–2597
Acknowledgments
This study was supported by the National Nature Science Foundation of China (Grant 31140019), Nature Science Foundation of Jiangxi Province (Grant 20122BAB205007) and Science and Technology Support Program of Nanchang city. The authors thank Molecular Center of the Second Affiliated Hospital of Nanchang University for their help in providing the equipment and technical support.
Conflict of interests
All authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ping Li and Xin Guo are the co-first authors.
Rights and permissions
About this article
Cite this article
Li, P., Guo, X., Lei, P. et al. PI3K/Akt/uncoupling protein 2 signaling pathway may be involved in cell senescence and apoptosis induced by angiotensin II in human vascular endothelial cells. Mol Biol Rep 41, 6931–6937 (2014). https://doi.org/10.1007/s11033-014-3580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3580-0