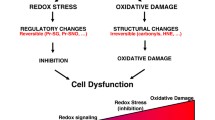
Abstract
The increased longevity in modern societies raised the attention to biological interventions that could promote a healthy aging. Mitochondria are main organelles involved in the production of adenosine triphosphate (ATP), the energetic substrate for cellular biochemical reactions. The production of ATP occurs through the oxidative phosphorylation of intermediate substrates derived from the breakdown of lipids, sugars, and proteins. This process is coupled to production of oxygen reactive species (ROS) that in excess will have a deleterious role in cellular function. The damage promoted by ROS has been emphasized as one of the main processes involved in senescence. In the last decades, the discovery of specialized proteins in the mitochondrial inner membrane that promote the uncoupling of proton flux (named uncoupling proteins–UCPs) from the ATP synthase shed light on possible mechanisms implicated in the buffering of ROS and consequently in the process of aging. UCPs are responsible for a physiological uncoupling that leads to decrease in ROS production inside the mitochondria. Thus, induction of uncoupling through UCPs could decrease the cellular damage that occurs during aging due to excess of ROS. This review will focus on the evidence supporting these mechanisms.
Similar content being viewed by others
References
Andrews ZB, Horvath TL (2009) Uncoupling protein-2 regulates lifespan in mice. Am J Physiol Endocrinol Metab 296:E621–E627
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70:200–214
Arechaga I, Ledesma A, Rial E (2001) The mitochondrial uncoupling protein UCP1: a gated pore. IUBMB Life 52:165–173
Arsenijevic D, Onuma H, Pecqueur C et al (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26:435–439
Asami DK, McDonald RB, Hagopian K et al (2008) Effect of aging, caloric restriction, and uncoupling protein 3 (UCP3) on mitochondrial proton leak in mice. Exp Gerontol 43:1069–1076
Barja G (1998) Mitochondrial free radical production and aging in mammals and birds. Ann N Y Acad Sci 854:224–238
Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembranes 31:347–366
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Bevilacqua L, Ramsey JJ, Hagopian K et al (2004) Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab 286:E852–E861
Bevilacqua L, Ramsey JJ, Hagopian K et al (2005) Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab 289:E429–E438
Bishop NA, Guarente L (2007) Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet 8:835–844
Boss O, Samec S, Paoloni-Giacobino A et al (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett 408:39–42
Brand MD (2000) Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol 35:811–820
Caldeira da Silva CC, Cerqueira FM, Barbosa LF et al (2008) Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7:552–560
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Conti B, Sanchez-Alavez M, Winsky-Sommerer R et al (2006) Transgenic mice with a reduced core body temperature have an increased life span. Science 314:825–828
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
Diano S, Urbanski HF, Horvath B et al (2000) Mitochondrial uncoupling protein 2 (UCP2) in the nonhuman primate brain and pituitary. Endocrinology 141:4226–4638
Echtay KS, Roussel D, St-Pierre J et al (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99
Echtay KS, Murphy MP, Smith RA et al (2002) Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem 277:47129–47135
Feng J, Bussière F, Hekimi S (2001) Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell 1:633–644
Fleury C, Neverova M, Collins S et al (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15:269–272
Fridell YW, Sánchez-Blanco A, Silvia BA, Helfand SL (2005) Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab 1:145–152
Gong DW, He Y, Karas M, Reitman M (1997) Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists, and leptin. J Biol Chem 272:24129–24132
Holzenberger M, Dupont J, Ducos B et al (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187
Horvath TL, Warden CH, Hajos M et al (1999) Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J Neurosci 19:10417–10427
Kim-Han JS, Reichert SA, Quick KL, Dugan LL (2001) BMCP1: a mitochondrial uncoupling protein in neurons which regulates mitochondrial function and oxidant production. J Neurochem 79:658–668
Klingenberg M, Appel M (1989) The uncoupling protein dimer can form a disulfide cross-link between the mobile C-terminal SH groups. Eur J Biochem 180:123–131
Kowaltowski AJ, Costa AD, Vercesi AE (1998) Activation of the potato plant uncoupling mitochondrial protein inhibits reactive oxygen species generation by the respiratory chain. FEBS Lett 425:213–216
Krauss S, Zhang CY, Lowell BB (2005) The mitochondrial uncoupling-protein homologues. Nature Rev Mol Cell Biol 6:248–261
Lebovitz RM, Zhang H, Vogel H et al (1996) Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA 93:9782–9787
Lin SJ, Kaeberlein M, Andalis AA et al (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418:344–348
Lockie SH, Müller TD, Tschöp MH (2009) Coupled with uncouplers: the curious case of lifespan. Am J Physiol Endocrinol Metab 296:E619–E620
Mao W, Yu XX, Zhong A et al (1999) UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett 443:326–330
McDonald RB, Walker KM, Warman DB et al (2008) Characterization of survival and phenotype throughout the life span in UCP2/UCP3 genetically altered mice. Exp Gerontol 43:1061–1068
Miroux B, Frossard V, Raimbault S et al (1993) The topology of the brown adipose tissue mitochondrial uncoupling protein determined with antibodies against its antigenic sites revealed by a library of fusion proteins. EMBO J 12:3739–3745
Murphy MP, Echtay KS, Blaikie FH et al (2003) Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem 278:48534–48545
Nègre-Salvayre A, Hirtz C, Carrera G et al (1997) A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11:809–815
Nicholls DG, Locke RM (1984) Thermogenic mechanisms in brown fat. Physiol Rev 64:1–64
Pecqueur C, Alves-Guerra MC, Gelly C et al (1998) Distribution of the uncoupling protein 2 mRNA in the mouse brain. J Comp Neurol 397:549–560
Pecqueur C, Alves-Guerra MC, Gelly C et al (2001) Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 276:8705–8712
Richard D, Rivest R, Huang Q et al (1998) Distribution of the uncoupling protein 2 mRNA in the mouse brain. J Comp Neurol 397:549–560
Richard D, Clavel S, Huang Q et al (2001) Uncoupling protein 2 in the brain: distribution and function. Biochem Soc Trans 29:812–817
Ricquier D, Bouillaud F, Miroux B (2001) Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem 276:8705–8712
Sanchis D, Fleury C, Chomiki N et al (1998) BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. J Biol Chem 273:34611–34615
Sivitz WI, Fink BD, Donohoue PA (1999) Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology 140:1511–1519
Skulachev VP (1998) Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1363:100–124
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Speakman JR, Talbot DA, Selman C et al (2004) Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3:87–95
Tahara EB, Barros MH, Oliveira GA et al (2007) Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J 21:274–283
Vidal-Puig A, Solanes G, Grujic D et al (1997) UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun 235:79–82
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Yamada S, Isojima Y, Yamatodani A, Nagai K (2000) Uncoupling protein 2 influences dopamine secretion in PC12h cells. J Neurochem 87:461–469
Zhang CY, Baffy G, Perret P et al (2001) Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105:745–755
Acknowledgements
MOD was partially supported by a fellowship from the Conselho Nacional de Pesquisa (CNPq), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dietrich, M.O., Horvath, T.L. The role of mitochondrial uncoupling proteins in lifespan. Pflugers Arch - Eur J Physiol 459, 269–275 (2010). https://doi.org/10.1007/s00424-009-0729-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0729-0