Abstract
Aims/hypothesis
Increased oxidative stress in vascular smooth muscle cells (VSMCs) has been implicated in the pathogenesis of accelerated atherosclerosis in patients with diabetes mellitus. Uncoupling protein 2 (UCP-2) is an important regulator of intracellular reactive oxygen species (ROS) production. We hypothesised that UCP-2 functions as an inhibitor of the atherosclerotic process in VSMCs.
Methods
Overexpression of human UCP-2 was performed in primary cultured human VSMCs (HVSMCs) via adenovirus-mediated gene transfer. Its effects on ROS production, AP-1 activity, plasminogen activator inhibitor 1 (PAI-1) gene expression, and cellular proliferation and migration were measured in response to high glucose and angiotensin II (Ang II) concentrations, two major factors in the pathogenesis of atherosclerosis in patients with diabetes and hypertension. Mitochondrial membrane potential and NAD(P)H oxidase activity were also measured.
Results
High glucose and Ang II caused transient mitochondrial membrane hyperpolarisation. They also significantly stimulated ROS production, NAD(P)H oxidase activity, mitochondrial membrane potential, AP-1 activity, PAI-1 mRNA expression, and proliferation and migration of HVSMCs. Adenovirus-mediated transfer of the UCP-2 gene reversed all of these effects.
Conclusions/interpretation
The present study demonstrates that UCP-2 can modify atherosclerotic processes in HVSMCs in response to high glucose and Ang II. Our data suggest that agents increasing UCP-2 expression in vascular cells may help prevent the development and progression of atherosclerosis in patients with diabetes and hypertension.
Similar content being viewed by others
Introduction
The risk of cardiovascular disease is increased in patients with diabetes mellitus, especially when diabetes is combined with hypertension [1, 2]. The proliferation and migration of vascular smooth muscle cells (VSMCs), leading to excessive accumulation of VSMCs within the arterial intima layer, are key processes in atherosclerosis [3, 4]. VSMCs within the intima produce various atherogenic mediators including plasminogen activator inhibitor 1 (PAI-1) [5]. This protein is the main physiological inhibitor of tissue-type plasminogen activator and is considered to be the most important inhibitor of fibrinolysis [6]. Moreover, in VSMCs, PAI-1 promotes neointimal formation after vascular injury [7, 8].
It is now well established that an increase in oxidative stress in vascular cells plays a key role in the pathogenesis of atherosclerosis [9, 10]. High glucose and angiotensin II (Ang II) concentrations, which contribute to the pathogenesis of atherosclerosis in patients with diabetes and hypertension respectively, lead to intracellular oxidative stress [11, 12]. In previous studies, we demonstrated that oligodeoxynucleotide (ODN)-mediated inhibition of the redox-sensitive transcription factor activator protein 1 (AP-1) decreased high-glucose- and Ang-II-induced proliferation and PAI-1 gene expression in human VSMCs (HVSMCs) [13, 14]. These results support the concept that relatively high levels of oxidative stress in vascular cells induce atherogenic genes via redox-sensitive signalling pathways and transcription factors [10].
Although the relative contribution of the individual reactive oxygen species (ROS)-generating systems in the vasculature is still ambiguous, both cell membrane NAD(P)H oxidase and the mitochondrial electron-transport chain have been shown to play significant roles in the overproduction of ROS by Ang II [12, 15] and high glucose [16, 17]. UCP-2 is a newly identified member of the mitochondrial anion carrier family and shares 60% sequence identity with the well-known thermogenic UCP-1 from brown adipose tissue [18]. Several lines of evidence suggest that UCP-2 is involved in the control of ROS production by mitochondria [19–21]. More recently, a direct role for UCP-2 in the regulation of atherogenesis has been suggested by the observation that bone marrow transplantation from UCP-2-deficient mice to LDL-receptor-deficient mice markedly increased atherosclerotic lesion size [22]. However, there has been no previous investigation of UCP-2 function in VSMCs. Therefore, the aim of this study was to investigate the possible role of UCP-2 in the regulation of atherogenesis in VSMCs.
Materials and methods
Cell culture
HVSMCs were isolated from the thoracic aorta of organ transplantation donors by the explant method as described previously [14]. Tissue collection was approved by the ethics committee of the institution. Cells were cultured in DMEM (Gibco BRL, Gaithersburg, MD, USA) containing 20% fetal bovine serum (Hyclone, Logan, UT, USA). In each preparation, HVSMC purity was determined by positive staining with smooth-muscle-specific α-actin monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All the cells were used within passages 5 and 6. After reaching 90% confluency in 100-mm dishes, cells were serum starved for 24 h and exposed to either control, normal glucose (DMEM containing 10% serum and 5.5 mmol/l d-glucose) or conditioned medium (DMEM containing 10% serum and 22 mmol/l d-glucose).
Preparation of recombinant adenovirus
The cDNA encoding the full-length human UCP-2 was inserted into the HindIII/BamHI site of the pAd-YC2 shuttle vector [23]. Shuttle vectors containing human UCP-2 cDNA and the rescue vector pJM17 [24, 25] were co-transfected into human embryonic kidney 293 (HEK-293) cells, which were cultured on 24-well plates the day before transfection. After 12 to 15 days, recombinants were identified by PCR [23], following which they were amplified in HEK-293 cells, and purified and isolated using CsCl (Sigma, St Louis, MO, USA). The preparations were collected and desalted and titres were determined by measuring plaque counts. Control adenovirus not containing UCP-2 (Ad-Null) was prepared and identified by the same method.
Measurement of intracellular H2O2 production
HVSMCs were seeded into six-well plates containing cover glasses. After reaching 90% confluency, HVSMCs were incubated in serum-free media for 24 h. Cells treated with 6×106 plaque-forming units of adenovirus containing the UCP-2 gene (Ad-UCP-2) or Ad-Null were cultured for 48 h in media containing 2% fetal bovine serum. After exposure to Ang II (100 nmol/l; Sigma) for 4 h, 10 μmol/l 2′,7′-dichlorofluorescein diacetate (Sigma), a H2O2-sensitive fluorescent probe, were added and HVSMCs were cultured for 30 min. H2O2 production was quantified using Zeiss LSM 410 confocal scanning laser microscopy at an excitation wavelength of 488 nm and an emission wavelength of 515 nm. Under the same imaging conditions, six images were analysed for each condition using the NIH program.
Measurement of NAD(P)H oxidase activity
HVSMCs were washed twice with PBS, then lysed with lysis buffer (50 mmol/l Tris–HCl, 150 mmol/l NaCl, 1 mmol/l EDTA, 1% Triton X-100, 10% glycerol, 1 mmol/l PMSF, 1 mg/ml aprotinin, 1 mg/ml leupeptin) and incubated for 1 h on ice. The lysate was centrifuged at 12,000 g for 20 min and the supernatant was saved. Protein content was determined using the Bradford method (Bio-Rad, Hercules, CA, USA). NAD(P)H oxidase activity was measured by lucigenin chemiluminescence [26].
Measurement of mitochondrial membrane potential
The degree of polarisation of the mitochondria was determined by loading with tetramethylrhodamine (TMRM; Molecular Probes, Eugene, OR, USA) as described previously [27]. Forty-eight hours after infecting cells with Ad-UCP-2 or Ad-Null, cells were seeded to 96-well culture plates and exposed to either control, Ang II (100 nmol/l) or high glucose (22 mmol/l d-glucose) conditions for the given time periods. Cells were incubated with 50 nmol/l TMRM for 20 min at 37°C and then rinsed with Hanks’ balanced salt solution (10 mmol/l HEPES, pH 7.4, 150 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 1.8 mmol/l CaCl2). The plate was immediately placed in a microplate spectrofluorometer (SPECTRAmax GEM-INI-XS; Molecular Devices, Sunnyvale, CA, USA), and the absorbance of TMRM was determined by 485-nm excitation and 590-nm emission.
Electrophoretic mobility shift assay
Nuclear extracts were prepared from HVSMCs. DNA probes were labelled using [γ-32P]ATP and T4 polynucleotide kinase. Following end-labelling, 32P-labelled ODNs were purified using a NAP-5 column. Protein–DNA binding reactions were performed as described previously [14]. Experimental conditions for competition studies were identical, except that appropriate competitor ODNs were added to the reaction mixtures in 50- to 100-fold molar excess before nuclear extracts were added.
RT-PCR
Aliquots of total RNA (1 μg) from each sample were reverse-transcribed into cDNA according to the instructions of the First Strand cDNA Synthesis Kit manufacturer (MBI, Vilnius, Lithuania). Equal amounts of the reverse transcriptional products were subjected to PCR amplification. The primers used for amplification of human UCP-2 were 5′-ATCTCCTGGGACGTAGCAG-3′ (forward) and 5′-CCAGCTCAGCACAGTTTGAC-3′ (reverse). The mRNA levels were normalised with β-actin mRNA levels.
Northern blot analysis
The radiolabelled probes used for northern blot analysis were prepared by random primer labelling with [α-32P]dCTP using a random primer labelling kit (Amersham, Arlington Heights, IL, USA). Northern blot analysis was performed as described previously [14].
Cell growth assay
HVSMCs were seeded on 96-well tissue culture plates. At 30% confluency, they were rendered quiescent by incubation for 24 h in serum-free medium. After treatment with 6×106 plaque-forming units of Ad-UCP-2 or Ad-Null, Ang II (100 nmol/l) was added to the wells and cells were incubated at 37°C for a further 4 h. The cell proliferation rate was determined using a WST cell counting kit (Wako, Osaka, Japan).
Cell migration assay
HVSMCs were seeded on six-well plates at 90% confluency and were incubated in serum-free medium for 24 h. They were then treated with 6×106 plaque-forming units of Ad-UCP-2 or Ad-Null and were wounded by razor scraping. After rinsing with PBS, Ang II (100 nmol/l) was added to the wells. Cells were allowed to migrate for 48 h and were then stained with haematoxylin and eosin. The extent of migration was calculated by direct cell counting.
Statistical analysis
Results are expressed as means±SEM. Analysis of variance with a subsequent Duncan’s test was used to determine statistical significance in multiple comparisons. A p value of less than 0.05 was considered statistically significant. All experiments were performed at least three times.
Results
Expression of UCP-2 mRNA in HVSMCs and up-regulation by angiotensin II and high glucose
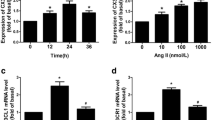
We initially examined whether UCP-2 mRNA is basally expressed in HVSMCs and whether the level of expression changes in response to high glucose and Ang II. RT-PCR indicated that primary cultured HVSMCs do express UCP-2 mRNA, and that its expression level was increased by both Ang II and high glucose (Fig. 1a, p<0.01 and p<0.001 respectively).
Expression of UCP-2 mRNA (a) and adenovirus-mediated overexpression of UCP-2 (b) in HVSMCs. a NG, normal glucose (5.5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l). **p<0.01 vs NG; ***p<0.001 vs NG; # p<0.05 vs HG. b Null, Ad-Null; p.f.u., plaque-forming units. *p<0.05 vs Null; **p<0.01 vs Null
Adenovirus-mediated UCP-2 gene overexpression in HVSMCs
To determine whether UCP-2 reduces the production of ROS, we used adenoviral infection to overexpress UCP-2 in HVSMCs. HVSMCs infected with adenoviruses containing UCP-2 (Ad-UCP-2) cDNA at doses of 3, 6 and 12×106 plaque-forming units/ml exhibited four-, 42- and 69-fold increases in UCP-2 mRNA, respectively, compared with cells infected with Ad-β-gal (Fig. 1b). We used 6×106 plaque-forming units/ml of Ad-UCP-2 in subsequent experiments.
Suppression of H2O2 production by Ad-UCP-2
Ang II (100 nmol/l) and high glucose (22 mmol/l) increased the production of H2O2 compared with that observed under normal glucose (5.5 mmol/l) conditions (p<0.01 for each). Transfection of Ad-UCP-2 significantly reduced H2O2 production induced by Ang II or high glucose, whereas transfection of Ad-Null did not influence this parameter (Fig. 2).
Effect of Ad-UCP-2 on H2O2 production in response to high glucose and Ang II. Quiescent HVSMCs were infected with 6×106 of Ad-Null or Ad-UCP-2. Cells were maintained in media containing normal glucose or high glucose with 2% FBS for 2 days, after which they were processed for confocal microscopic examination using the oxidant-sensitive probes 2′,7′-dichlorofluorescein diacetate. Fully confluent fields of HVSMCs were randomly chosen and fluorescence was quantified using the NIH Image program. a NG+Ad-Null; b NG+Ang II+Ad-Null; c NG+Ang II+Ad-UCP-2; d HG+Ad-Null; e HG+Ang II+Ad-Null; f HG+Ang II+Ad-UCP-2. NG, normal glucose (5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l). Values are means±SEM of three independent experiments (g). **p<0.01 vs NG; §§ p<0.01 vs NG+Ang II; # p<0.05 vs HG; ## p<0.01 vs HG+Ang II
Effect of Ad-UCP-2 on NAD(P)H oxidase
Both high glucose and Ang II increased the NAD(P)H oxidase activity compared with that observed under normal glucose conditions (p<0.05 for each). Transfection of Ad-UCP-2 significantly reduced NAD(P)H oxidase activity (Fig. 3a).
Effects of Ad-UCP-2 on NAD(P)H oxidase activity (a) and mitochondrial membrane potential in response to high glucose (b) and Ang II (c). a HVSMCs were infected with 6×106 of Ad-Null or Ad-UCP-2 for 44 h under normal glucose or high glucose conditions, with or without Ang II for 4 h. NG, normal glucose (5.5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l); Null, Ad-Null; UCP-2, Ad-UCP-2. Values are means±SEM of three independent experiments. *p<0.05 vs NG; § p<0.05 vs NG+Ang II; # p<0.05 vs HG; ## p<0.01 vs HG+Ang II. b, c HVSMCs were infected with 6×106 of Ad-Null or Ad-UCP-2 for 48 h and exposed to high glucose or Ang II. *p<0.05 vs basal; § p<0.05 vs Ad-Null
Effect of Ad-UCP-2 on the mitochondrial membrane potential
The changes in the membrane potential in response to high glucose and Ang II were determined over a 24- or 3-h period respectively. There was a rapid increase in the membrane potential, which peaked at 3 h following exposure to glucose (Fig. 3b). The membrane potential in response to Ang II also increased and peaked at 30 min (Fig. 3c). The membrane potentials then decreased and were lower than those of the control cells. Overexpression of UCP-2 completely prevented glucose- and Ang-II-induced changes in the membrane potential observed in Ad-Null-infected cells (Fig. 3b, c).
Effect of Ad-UCP-2 on AP-1 activity
Intracellular oxidative stress activates the redox-sensitive transcription factor AP-1 to influence various cellular functions. Increased AP-1 DNA binding activity in response to high glucose, in the presence or absence of Ang II, was significantly decreased by Ad-UCP-2, whereas Ad-Null had no effect (Fig. 4, p<0.01).
Effect of Ad-UCP-2 on AP-1 DNA binding activity. 6 μg of nuclear extract were incubated with radiolabelled AP-1 probe. a A typical example of a gel shift assay is shown for HVSMCs transfected with Ad-UCP-2. b Bars represent fold increase compared with normal glucose. NG, normal glucose (5.5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l); Null, Ad-Null; UCP-2, Ad-UCP-2 for adenoviral vector treatment. Values are means±SEM of three independent experiments. **p<0.01 vs NG; §§ p<0.01 vs HG; ### p<0.001 vs NG; ## p<0.01 vs HG+Ang II
Effect of Ad-UCP-2 on PAI-1 mRNA expression
Ang II and high glucose significantly increased PAI-1 mRNA expression (Fig. 5a, p<0.01 for each). Increased PAI-1 mRNA expression by Ang II or high glucose was reduced to the level observed under normal glucose conditions by the transfer of Ad-UCP-2, whereas Ad-Null did not affect PAI-1 mRNA expression. The reduction of PAI-1 mRNA expression was dose dependent (Fig. 5b).
Effect of Ad-UCP-2 on PAI-1 expression. NG, normal glucose (5.5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l). Values are means±SEM of three independent experiments. a HVSMCs were infected with either 6×106 of Ad-Null or Ad-UCP-2. HVSMCs were stimulated with Ang II or high glucose, with or without Ang II. Total RNA was extracted from HVSMCs. 10 μg of total RNA were hybridised with PAI-1 probe. RNA quantity was normalised by 18S rRNA. **p<0.01 vs NG; ***p<0.001 vs NG+Ang II; # p<0.05 vs HG; ## p<0.01 vs HG+Ang II. b Dose-dependent effect of Ad-UCP-2 on PAI-1 expression. HVSMCs were infected with indicated dose of Ad-UCP-2. **p<0.01 vs NG; *p<0.05 vs NG+Ang II; §§ p<0.01 vs NG+Ang II; ## p<0.01 vs HG. p.f.u., plaque-forming units
Effect of Ad-UCP-2 on proliferation and migration of HVSMCs
Ang II and high glucose stimulated the proliferation of primary cultured HVSMCs (p<0.01 for each). Transfection of Ad-UCP-2 significantly inhibited Ang-II- or high-glucose-mediated cell proliferation (Fig. 6a, p<0.01 for each).
Effect of Ad-UCP-2 on cell proliferation (a) and migration (b) in HVSMCs. NG, normal glucose (5 mmol/l); HG, high glucose (22 mmol/l); Ang II, angiotensin II (100 nmol/l). Values are means±SEM of three independent experiments. a Cells were infected with 6×106 of Ad-Null or Ad-UCP-2. Three days after infection, growth was measured using a WST kit. Optical density (OD) values indicate growth activity of cells. **p<0.01 vs NG; §§ p<0.01 vs NG+Ang II; ## p<0.01 vs HG; ++ p<0.01 vs HG+Ang II. b Cells were infected with 6×106 of Ad-Null or Ad-UCP-2. Two days after infection, migration was measured by direct cell counting. **p<0.01 vs NG; §§ p<0.01 vs NG+Ang II; # p<0.05 vs HG; ## p<0.01 vs HG+Ang II
Similarly, Ang II and high glucose stimulated the migration of cultured primary HVSMCs compared with that observed under normal glucose conditions (p<0.01 for each). This increase in HVSMC migration in response to Ang II or high glucose was markedly decreased by Ad-UCP-2 but not affected by Ad-Null (Fig. 6b, p<0.01 for each).
Discussion
We demonstrate here that UCP-2 is expressed in HVSMCs and is up-regulated by Ang II and/or high glucose. Adenoviral transfer of the UCP-2 gene to HVSMCs profoundly reduced ROS generation, AP-1 activity and mRNA expression of PAI-1, and also inhibited proliferation and migration of HVSMCs in response to high glucose and Ang II.
Hyperglycaemia and high-plasma Ang II concentrations are thought to play major roles in the pathogenesis of atherosclerosis in patients with diabetes and hypertension. Accumulating evidence indicates that high glucose and Ang II increase intracellular ROS production [12, 16, 17]. Consistent with these previous results, we found that high glucose and Ang II increase ROS generation, as well as the activity of the redox-sensitive transcription factor AP-1, and PAI-1 expression in HVSMCs. We also found that high glucose and Ang II increase proliferation and migration of HVSMCs. We previously demonstrated that inhibition of AP-1 by a sequence-specific decoy ODN inhibited high-glucose- and Ang-II-induced cell proliferation and PAI-1 gene expression in primary cultured HVSMCs [13, 14]. When these results are combined with our current observations, it appears that high glucose and Ang II stimulate ROS generation in HVSMCs and that subsequent activation of AP-1 is responsible for increased PAI-1 expression as well as cellular proliferation and migration.
In this study, overexpression of the UCP-2 gene inhibited high-glucose- and Ang-II-stimulated AP-1 activity, PAI-1 expression, and proliferation and migration of HVSMCs. These results suggest that the effects of UCP-2 on HVSMC function are via the inhibition of AP-1 activity. However, direct inhibition of AP-1 by UCP-2 is less likely. The human UCP-2 promoter region has AP-1 binding sites near the transcription initiation site [28], which strongly suggests that transcription of UCP-2 is induced through activation of AP-1. High glucose and Ang II may increase UCP-2 expression by intracellular ROS-induced activation of AP-1. Increases in UCP-2 may in turn decrease intracellular ROS and AP-1 activity and may limit cellular proliferation and PAI-1 gene expression of HVSMCs. In this regard, high-glucose- or Ang-II-induced UCP-2 overexpression may be a compensatory mechanism for reducing intracellular ROS and limiting detrimental cellular effects of AP-1.
In cultured neuronal cells, high glucose induces initial hyperpolarisation followed by depolarisation of the inner mitochondrial membrane potential, which is associated with increased generation of ROS [27, 29]. Consistent with these studies, we found that high glucose and Ang II caused mitochondrial membrane hyperpolarisation followed by depolarisation. As expected, UCP-2 overexpression prevented these processes as well as ROS production [27]. Cell membrane NAD(P)H oxidase is another major source of ROS generation in VSMCs. Consistent with previous observations [12, 15, 26], both high glucose and Ang II increased NAD(P)H oxidase activity. Quite unexpectedly, we also found that UCP-2 overexpression significantly decreased high-glucose- and Ang-II-induced increases in NAD(P)H oxidase activity. The mechanism by which UCP-2 overexpression decreases NAD(P)H oxidase activity is presently unknown, but may be due to its effect on the cellular NADH/NAD+ redox state. Bassenger et al. reported that reduction of the NADH concentration : NAD+ concentration ratio (NADH : NAD+ redox) by pyruvate infusion dose-dependently inhibited NAD(P)H oxidase activity and ROS generation in perfused guinea pig hearts [30]. Therefore, it can be assumed that high-glucose- and Ang-II-induced mitochondrial membrane hyperpolarisation, which reflects impairment of mitochondrial electron transfer and respiration, would increase NAD(P)H oxidase activity by increasing the cytosolic NADH : NAD+ ratio. Conversely, normalisation of mitochondrial membrane potential by UCP-2 overexpression would decrease NAD(P)H oxidase activity by reducing the cytosolic NADH : NAD+ ratio. The mechanism by which Ang II induces mitochondrial membrane hyperpolarisation is yet to be investigated.
In summary, the present study shows that Ang-II- and high-glucose-induced ROS production up-regulates UCP-2 expression in HVSMCs. Adenovirus-mediated transfer of the UCP-2 gene diminished the expression of PAI-1 mRNA as well as cell proliferation and migration under high glucose and Ang II conditions. This study presents the possibility that the agents increasing UCP-2 expression in the vasculature may help prevent the development and progression of atherosclerosis in patients with diabetes and hypertension.
Abbreviations
- Ang II:
-
angiotensin II
- AP-1:
-
activator protein 1
- HVSMCs:
-
human vascular smooth muscle cells
- ODN:
-
oligodeoxynucleotide
- PAI-1:
-
plasminogen activator inhibitor 1
- ROS:
-
reactive oxygen species
- TMRM:
-
tetramethylrhodamine
- UCP:
-
uncoupling protein
- VSMCs:
-
vascular smooth muscle cells
References
Sowers JR, Epstein M, Frohlich ED (2001) Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37:1053–1059
Jones DW, Chambless LE, Folsom AR et al (2002) Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987–1997. Arch Intern Med 162:2565–2571
Ross R (1995) Cell biology of atherosclerosis. Annu Rev Physiol 57:791–804
Newby AC, Zaltsman AB (1999) Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res 41:345–360
Dzau VJ, Braun-Dullaeus RC, Sedding DG (2002) Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 8:1249–1256
Schneiderman J, Sawdey MS, Keeton MR et al (1992) Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A 89:6998–7002
Ploplis VA, Cornelissen I, Sandoval-Cooper MJ, Weeks L, Noria FA, Castellino FJ (2001) Remodeling of the vessel wall after copper-induced injury is highly attenuated in mice with a total deficiency of plasminogen activator inhibitor-1. Am J Pathol 158:107–117
Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP (2002) Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol 22:934–939
Parker RA, Sabrah T, Cap M, Gill BT (1995) Relation of vascular oxidative stress, alpha-tocopherol, and hypercholesterolemia to early atherosclerosis in hamsters. Arterioscler Thromb Vasc Biol 15:349–358
Kunsch C, Medford RM (1999) Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85:753–766
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW (1994) Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74:1141–1148
Ahn JD, Morishita R, Kaneda Y et al (2002) Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res 90:1325–1332
Ahn JD, Morishita R, Kaneda Y et al (2001) Transcription factor decoy for activator protein-1 (AP-1) inhibits high glucose- and angiotensin II-induced type 1 plasminogen activator inhibitor (PAI-1) gene expression in cultured human vascular smooth muscle cells. Diabetologia 44:713–720
Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM (1998) Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 83:952–959
Du XL, Edelstein D, Rossetti L et al (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97:12222–12226
Nishikawa T, Edelstein D, Du XL et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790
Boss O, Hagen T, Lowell BB (2000) Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes 49:143–156
Arsenijevic D, Onuma H, Pecqueur C et al (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26:435–439
Duval C, Negre-Salvayre A, Dogilo A, Salvayre R, Penicaud L, Casteilla L (2002) Increased reactive oxygen species production with antisense oligonucleotides directed against uncoupling protein 2 in murine endothelial cells. Biochem Cell Biol 80:757–764
Li LX, Skorpen F, Egeberg K, Jorgensen IH, Grill V (2001) Uncoupling protein 2 participates in cellular defence against oxidative stress in clonal β-cells. Biochem Biophys Res Commun 282:273–277
Blanc J, Alves-Guerra MC, Esposito B et al (2003) Protective role of uncoupling protein 2 in atherosclerosis. Circulation 107:388–390
Choi YK, Kim YJ, Park HS et al (2003) Suppression of glomerulosclerosis by adenovirus-mediated IL-10 expression in the kidney. Gene Ther 10:559–568
Graham FL, van der Eb AJ (1973) A new technique for the assay of infectivity of adenovirus 5 DNA. Virology 52:456–467
McGrory MJ, Bautista DS, Graham FL (1988) A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology 163:614–617
Lee HS, Son SM, Kim YK, Hong KW, Kim CD (2003) NAD(P)H oxidase participates in the signaling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci 72:2719–2730
Vincent AM, Olzmann JA, Brownlee M, Sivitz WI, Russell JW (2004) Uncoupling proteins prevent glucose-induced neuronal oxidative stress and programmed cell death. Diabetes 53:726–734
Tu N, Chen H, Winnikes U et al (1999) Molecular cloning and functional characterization of the promoter region of the human uncoupling protein-2 gene. Biochem Biophys Res Commun 265:326–334
Russell JW, Golovoy D, Vincent AM et al (2002) High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16:1738–1748
Bassenge E, Sommer O, Schwemmer M, Bunger R (2000) Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am J Physiol Heart Circ Physiol 279:H2431–H2438
Acknowledgements
This study was supported by a National Research Laboratory grant from the Korean Ministry of Science and Technology (M1040000000804J000000810).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Park, JY., Park, KG., Kim, HJ. et al. The effects of the overexpression of recombinant uncoupling protein 2 on proliferation, migration and plasminogen activator inhibitor 1 expression in human vascular smooth muscle cells. Diabetologia 48, 1022–1028 (2005). https://doi.org/10.1007/s00125-005-1712-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1712-8