Abstract
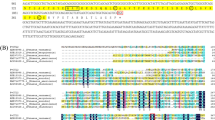
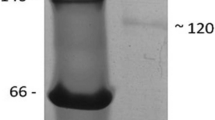
The β-1,3-d-glucan binding protein (βGBP), one kind of the pattern recognition proteins (PRPs), was cloned and characterized from the Chinese Shrimp Fenneropenaeus chinensis, and named as FcβGBP-HDL. The results indicated that the full length cDNA of 6713 bp had an open reading frame encoded a polypeptide of 2139 amino acids with two glucanase-like motifs and one RGD motif, while without signal peptide. The calculated molecular mass of mature protein was 240.7 kDa and theoretical pI was 5.95. Sequence comparison of the deduced amino acid sequence of FcβGBP-HDL showed varied identity of 88, 54 and 53% with those of Litopenaeus vannamei βGBP-HDL, Pacifastacus leniusculus βGBP, and Pontastacus leptodactylus βGBP, respectively. qRT-PCR analysis indicated that FcβGBP-HDL was expressed in intestines, hepatopancreas, muscle, gill and hemocytes, and its profile was modified post WSSV challenge. The differential expressions of FcβGBP-HDL in different tissues post WSSV challenge suggested that FcβGBP-HDL might play an important role in shrimp immune and perform differently in different tissues. These data would be helpful to better understand the WSSV-resistance mechanism of farming shrimp.






Similar content being viewed by others
References
Gross PS, Bartlett TC, Browdy CL, Chapman RW, Warr GW (2001) Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific white shrimp, Litopenaeus vannamei, and the Atlantic white shrimp, L. setiferus. Dev Comp Immunol 25:565–577
Smith VJ, Chisholm JRS (1992) Non-cellular immunity in crustaceans. Fish Shellfish Immunol 2:1–31
Zhang R, Cho HY, Kim HS (2003) Characterization and properties of a 1,3-β-d-glucan pattern recognition protein of Tenebrio molitor larvae that is specifically degraded by serine protease during prophenoloxidase activation. J Biol Chem 278(43):42072–42079
Lackie AM (1988) Hemocyte behavior. Adv Insect Physiol 21:85–178
Hoffmann JA, Reichart JM, Hetru C (1996) Innate immunity in higher insects. Curr Opin Immunol 8:8–13
Iwanaga S, Kawabata S, Miura Y, Seki N, Shigenaga T, Muta T (1994) Clotting cascade in the immune response of horseshoe crab. In: Hoffmann J, Janeway CA Jr, Natori S (eds) Phylogenetic perspectives in immunity: the insect host defense. R. G. Landes Company Biomedical Publishers, Austin, pp 79–96
Sritunyalucksana K, Soderhall K (2000) The proPO and clotting system in crustaceans. Aquaculture 191:53–59
Mellroth P, Karlsson J, Steiner H (2003) A scavenger function for a Drosophila peptidoglycan recognition protein. J Biol Chem 278:7059–7064
Takehana A, Katsuyama T, Yano T, Oshima Y, Takada H, Aigaki T, Kurata S (2002) Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc Natl Acad Sci USA 99:13705–13710
Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA (2002) Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416:644–648
Duvic B, Soderhall K (1993) β-1,3-Glucan-binding proteins from plasma of the freshwater crayfishes Astacus astacus and Procambarus clarkii. J Crust Biol 13:403–408
Romeo-Figueroa MG, Vargas-Requena C, Sotelo-Mundo RR, Vargas-Albores F, Higuera-Ciapara I, Soderhall K (2004) Molecular cloning of a β-glucan pattern-recognition lipoprotein from the white shrimp Penaeus (Litopenaeus) vannamei: correlations between the deduced amino acid sequence and the native protein structure. Dev Comp Immunol 28:713–726
Kim YS, Ryu JH, Han SJ, Choi KH, Nam KB, Jang IH, Lemaitre B, Brey PT, Lee WJ (2000) Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J Biol Chem 275:32721–32727
Roux MM, Pain A, Klimper KR, Dhar AY (2002) The lipopolysaccharide and β-1,3-glucan binding protein gene is upregulated in white spot virus-infected shrimp (Penaeus stylirostris). J Virol 76:7140–7149
Cheng W, Liu CH, Tsai CH, Chen JC (2005) Molecular cloning and characterization of a pattern recognition molecule, lipopolysaccharide-and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 18(4):297–310
Liu FS, Li FH, Dong B (2009) Molecular cloning and characterisation of a pattern recognition protein, lipopolysaccharide and β-1,3-glucan binding protein (LGBP) from Chinese shrimp Fenneropenaeus chinensis. Mol Biol Rep 36:471–477
Wang X, Fuchs JF, Infanger LC, Rocheleau TA, Hillyer JF, Chenchen C (2005) Mosquito innate immunity: involvement of β-1,3-glucan recognition protein in melanotic encapsulation immune responses in Armigeres subalbatus. Mol Biochem Parasitol 139:65–73
Soderhall K, Rogener W, Soderhall I, Newton RP, Ratcliffe NA (1988) The properties and purification of a Blaberus craniifer plasma protein which enhances the activation of haemocyte prophenoloxidase by a β-1,3-glucan. Insect Biochem 18:323–330
Ochiai M, Ashida M (1988) Purification of a β-1,3-glucan recognition protein in the prophenoloxidase activating system from the hemolymph of silkworm, Bombyx mori. J Biol Chem 263:12056–12062
Ma C, Kanost MR (2000) A β-1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J Biol Chem 275:7505–7514
Sritunyalucksana K, Lee SY, Soderhall K (2002) A beta-1,3-glucan binding protein from the black tiger shrimp, Penaeus monodon. Dev Comp Immunol 26:237–245
Jiménez-Vega F, Sotelo-Mundo RR, Ascencio F, Vargas-Albores F (2002) 1,3-β-d glucan binding protein (ΒGBP) from the white shrimp, Penaeus vannamei, is also a heparin binding protein. Fish Shellfish Immunol 13(3):171–181
Jayaraj SS, Thiagarajan R, Arumugam M, Mullainadhan P (2008) Isolation, purification and characterization of β-1,3-glucan binding protein from the plasma of marine mussel Perna viridis. Fish Shellfish Immunol 24(6):715–725
Chou HY, Huang CY, Wang CH (1995) Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeus shrimp in Taiwan. Dis Aquat Org 23:165–173
Liu F, Liu Y, Li F, Dong B, Xiang J (2005) Molecular cloning and expression profile of putative antilipopolysaccharide factor in Chinese shrimp (Fenneropenaeus chinensis). Mar Biotechnol 7:600–608
Chiou TT, Wu JL, Chen TT, Lu JK (2005) Molecular cloning and characterization of cDNA of penaeidinlike antimicrobial peptide from tiger shrimp (Penaeus monodon). Mar Biotechnol 7:119–127
Zhang J, Sun Y, Li F, Huang B, Xiang J (2010) Molecular characterization and expression analysis of chitinase (Fcchi-3) from Chinese shrimp, Fenneropenaeus chinensis. Mol Biol Rep 37(4):1913–1921
Dong B, Liu F, Gao H, Wang B, Xiang J (2009) cDNA cloning and gene expression pattern following bacterial challenge of peroxinectin in Chinese shrimp Fenneropenaeus chinensis. Mol Biol Rep 36(8):2333–2339
Mai WJ, Hu CQ (2009) Molecular cloning, characterization, expression and antibacterial analysis of a lysozyme homologue from Fenneropenaeus merguiensis. Mol Biol Rep 36(6):1587–1595
Ye X, Gao FY, Zheng QM, Bai JJ, Wang H, Lao HH, Jian Q (2009) Cloning and characterization of the tiger shrimp lysozyme. Mol Biol Rep 36(6):1239–1246
Gao H, Li F, Dong B, Zhang Q, Xiang J (2009) Molecular cloning and characterisation of prophenoloxidase (ProPO) cDNA from Fenneropenaeus chinensis and its transcription injected by Vibrio anguillarum. Mol Biol Rep 36(5):1159–1166
Jiang S, Qiu L, Zhou F, Huang J, Guo Y, Yang K (2007) Molecular cloning and expression analysis of a heat shock protein (Hsp90) gene from black tiger shrimp (Penaeus monodon). Mol Biol Rep 36(1):127–134
Jarasrassamee B, Supungul P, Panyim S, Klinbunga S, Rimphanichayakit V, Tassanakajon A (2005) Recombinant expression and characterization of five-domain Kazal-type serine proteinase inhibitor of black tiger shrimp (Penaeus monodon). Mar Biotechnol 7:46–52
Han F, Zhang XB (2007) Characterization of a ras-related nuclear protein (Ran protein) up-regulated in shrimp antiviral immunity. Fish Shellfish Immunol 23:937–944
Zhao ZY, Yin ZX, Xu XP (2009) A novel C-type lectin from the shrimp Litopenaeus vannamei possesses anti-white spot syndrome virus activity. J Virol 83(1):347–356
Wang B, Li F, Dong B (2006) Discovery of the genes in response to white spot syndrome virus (WSSV) infection in Fenneropenaeus chinensis through cDNA microarray. Mar Biotechnol (NY) 8:491–500
Vargas-Albores F, Jimenez-Vega F, Soderhall K (1996) A plasma protein isolated from brown shrimp (Penaeus californiensis) which enhances the activation of prophenoloxidase system by β-1,3-glucan. Dev Comp Immunol 20:299–306
Yepiz-Plascencia G, Vargas-Albores F, Jimenez-Vega F, Ruiz-Verdugo LM, Romo-Figueroa G (1998) Shrimp plasma HDL and β-glucan binding protein (BGBP): comparison of biochemical characteristics. Comp Biochem Physiol 121B:309–314
Vargas-Albores F, Jimenez-Vega F, Yepiz-Plascencia G (1997) Purification and comparison of β-1,3-glucan binding protein from white shrimp (Penaeus vannamei). Comp Biochem Physiol 116B:453–458
Lee SY, Wang R, Soderhall K (2000) A lipopolysaccharide- and β-1,3-glucan-binding protein from hemocytes of the freshwater crayfish Pacifastacus leniusculus. Purification, characterization and cDNA cloning. J Biol Chem 275:1337–1343
Zhu YX, Li Y (2005) Modern molecular biology, 2nd edn. Higher Education Press, Beijing, pp 88–92
Johansson MW (1999) Cell adhesion molecules in invertebrate immunity. Dev Comp Immunol 23:303–315
Cerenuis L, Liang Z, Duvic B, Keyser P, Hellman U, Tapio-Palva E, Iwanaga S, Soderhall K (1994) Structure and biological activity of a 1,3-β-d-glucan-binding protein in crustacean blood. J Biol Chem 269:29462–29467
Du XJ, Zhao XF, Wang JX (2007) Molecular cloning and characterization of a lipopolysaccharide and β-1,3-glucan binding protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 44:1085–1094
Su JG, Song LS, Xu W, Wu LT, Li HL, Xiang JH (2004) cDNA cloning and mRNA expression of the lipopolysaccharide and β-1,3-glucan binding protein gene from scallop Chlamys farreri. Aquaculture 239:69–80
Acknowledgments
This work was financially supported by National High-Tech R & D Program of China (863 program, No. 2006AA10A406 and No. 2010AA10A401) and by Jiangsu Key Laboratory of Marine Biotechnology program of China (No. 2008HS019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, X., Kong, J., Wang, Q. et al. Cloning and characterization of a β-1,3-glucan-binding protein from shrimp Fenneropenaeus chinensis . Mol Biol Rep 38, 4527–4535 (2011). https://doi.org/10.1007/s11033-010-0583-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0583-3