Abstract
An important research topic is the discovery of multifunctional compounds targeting different disease-causing components. This research aimed to design and synthesize a series of 2-aryl-6-carboxamide benzoxazole derivatives that inhibit cholinesterases on both the peripheral anionic and catalytic anionic sides. Compounds (7–48) were prepared from 4-amino-3-hydroxybenzoic acid in three steps. The Ellman test, molecular docking with Maestro, and molecular dynamics simulation studies with Desmond were done (Schrodinger, 12.8.117). Compound 36, the most potent compound among the 42 new compounds synthesized, had an inhibitory concentration of IC50 12.62 nM for AChE and IC50 25.45 nM for BChE (whereas donepezil was 69.3 nM and 63.0 nM, respectively). Additionally, compound 36 had docking values of − 7.29 kcal/mol for AChE and − 6.71 kcal/mol for BChE (whereas donepezil was − 6.49 kcal/mol and − 5.057 kcal/mol, respectively). Furthermore, molecular dynamics simulations revealed that compound 36 is stable in the active gorges of both AChE (average RMSD: 1.98 Å) and BChE (average RMSD: 2.2 Å) (donepezil had average RMSD: 1.65 Å and 2.7 Å, respectively). The results show that compound 36 is a potent, selective, mixed-type dual inhibitor of both acetylcholinesterase and butyrylcholinesterase. It does this by binding to both the catalytically active and peripheral anionic sites of cholinesterases at the same time. These findings show that target compounds may be useful for establishing the structural basis for new anti-Alzheimer agents.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a serious health problem that primarily affects the elderly and is the leading cause of dementia and behavioral disorders [1]. The neuronal cortex and hippocampus are the most affected areas of the brain when neurons degenerate in AD [2]. According to the World Health Organization (WHO), 50 million people are affected by this condition, and that number is expected to triple by 2050 [3].
The pathogenesis of AD is not completely understood; typical pathological signs include amyloid-β (Aβ) deposits, neurofibrillary tangles, oxidative stress, and decreased acetylcholine (ACh) levels in the brain [4]. Furthermore, it was discovered that ACh levels increased in patients because of an effective immune response designed to reduce cognitive and behavioral symptoms reported in AD patients [5]. So, over the last two decades, acetylcholinesterase (AChE) has been the focus of intensive pharmaceutical research, resulting in the development of several drugs currently in clinical use for the treatment of AD, with AChE inhibitors constituting many drugs approved by the FDA for the disease [6, 7].
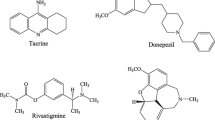
Since 1998, approximately 100 potential anti-Alzheimer drug candidates have been tested in clinical trials, but only 5 drugs have been approved for clinical use by the FDA (Fig. 1). Among these are tacrine [8] with an acridine ring, memantine [9] with an adamantyl group, rivastigmine [10] has an N-methyl-N-ethyl carbamate functional group, galantamine [11] with an azepane coupled with a benzofuran structure, and donepezil [12] with indene and piperidine. Tacrine, on the other hand, was withdrawn from the market due to the occurrence of severe adverse effects, such as hepatotoxicity, in a significant proportion of patients [13]. Currently, despite the availability of memantine, rivastigmine, galantamine, and donepezil on the market, several studies are being conducted to investigate the problems caused by the overuse of these medications and the numerous negative effects associated with their long-term use [14]. As a result, the development of new drugs for the treatment of AD has become a critical topic in recent years.
Researchers developed a multitarget-directed ligand (MTDL) strategy based on failed clinical trial drug candidates [15]. The evolutionary MTDL technique was used to discover that combining multiple drugs into a single formulation improves therapeutic effects. Several novel hybrid compounds with nanomolar cholinesterase inhibitory activity have been produced using synthetic methods for these drugs [16]. Recent synthetic studies have produced AChE inhibitors such as tacrine-benzofuran [17], tacrine-gallamine [18], tacrine-donepezil [18,19,20,21], tacrine-benzothiazole [22], and rivastigmine-benzoxazole [23]. Nonetheless, the discovered MTDL candidate compounds had adverse effects comparable to traditional AChE inhibitors and were shown to inhibit cholinesterase with various additional pharmacological features [24, 25].
Fragment-based approaches have been developed to efficiently produce multi-target drugs [26]. A fragment of cyclic amide, for example, is combined into a single pharmacophore molecule. The amino and carbonyl parts of the cyclic amide group have a unique H-bond network that allows them to inhibit several ATP-competitive kinases in AD [27]. Because recently developed hybrid compounds have side effects comparable to commercial AChE inhibitors, research has focused on developing selective, potent, and reversible AChE inhibitors. In this way, knowledge of protein-ligand interactions could be applied to the development of new drugs.
It is well understood that new ligands compatible with the active site of the AChE enzyme must be designed to create novel AChE inhibitors. Newly designed ligands are known to improve AChE enzyme activity by interacting with both the catalytic anionic site (CAS) and the peripheral anionic binding site (PAS) in the active sites [28]. The aromatic groups in compounds designed to inhibit AChE are chemically linked to the PAS region, whereas the groups bearing the basic center are bound to the CAS [29, 30]. Compounds with dual binding properties, such as donepezil, have an extremely potent AChE inhibition profile [30,31,32,33,34].
In addition to these, benzo-heterocyclic compounds have been developed, and numerous hybridizations with functional groups can be found in commercially available drugs. The benzoxazole ring structure was chosen as a new scaffold for AChE because it proved to be the most effective. Compound 1 is a benzoxazole derivative with a benzoxazole ring structure derived from thioflavin-T (Fig. 2). The N,N-dimethyl carbamate functional group, which mimics the carbamate in rivastigmine, is substituted into the benzoxazole structure of this molecule, and at a concentration of 58.2 nM, it inhibits AChE [23]. The pyrrole and piperidine groups are more effective than the piperazine analogs, with EC50 values of 1.03 nM, 1.35 nM, and 62.23 nM, respectively, in compound 2, where the p-position of the 2-phenyl benzoxazole derivatives are substituted with alicyclic amino-methyl derivatives [35]. It has also been discovered that replacing a 2-phenyl group at the p-position in the structure increases the activity of several benzoxazole derivatives. Both compound 3, which contains a piperazine-substituted amide at the 6-position, and compound 4, which contains a phenylacetamide derivative at the 6-position, were found to be highly potent AChE inhibitors (IC50: 3.67 nM and 38.36 nM, respectively) [36, 37]. Compounds 5 (3-morpholino methyl-2-thione benzoxazole, IC50: 44 nM) and 6 (3-(diethylamino) methyl-2-thione benzoxazole, IC50: 38 nM) were discovered to have potent AChE inhibition activity (Fig. 2) [38].
In this study, we created a series of inhibitors based on new scaffolds by incorporating functional groups that promote cationic, hydrogen bonding, and hydrophobic interactions with conserved and hot-spot residues. We rationally combined benzoxazole and aromatic ring systems with cyclic and acyclic nitrogen amide-containing moieties, which are logically required for effective enzyme-ligand interactions. We developed benzoxazole compounds with aromatic (phenyl, naphthyl, and biphenyl groups) and heteroaromatic (pyridine, pyrrole, furan, and thiophene) groups in the 2-position of benzoxazole to increase PAS binding potential for AChE in our fragment-based strategy. To increase the CAS binding potential for AChE, we modified the acyclic amides (dimethyl, diethyl, and dipropyl) and cyclic amides (pyrrolidine, piperidine, and morpholine) in the 6-position of benzoxazole (Fig. 3).
Results and discussion
Chemistry
In three steps, the target compounds (7–48) were synthesized from 4-amino-3-hydroxybenzoic acid. In the first step, 4-amino-3-hydroxy-benzoic acid was refluxed in methanol for 12 h with a few drops (5% mol) of concentrated H2SO4. 4-Amino-3-hydroxybenzoate was successfully obtained in 98% yield after the necessary purification processes. The reaction of 4-amino-3-hydroxybenzoate with related aromatic carboxaldehyde derivatives yielded 2-aryl benzoxazole derivatives in the second step (details are indicated in supporting information). The target compounds were obtained in very high yields in the final step by treating 2-aryl benzoxazole derivatives in DCM and the presence of AlCl3 with cyclic or linear aliphatic secondary amines (pyrrolidine, piperidine, morpholine or dimethylamine, diethylamine, and dipropylamine) (Scheme 1).
Enzyme inhibition study
In the treatment of AD, cholinesterase (ChE) inhibitors are frequently utilized. AChE is a major target for new drug designs for the treatment of AD [39, 40]. Current research indicates that BChE plays a significant role in fiber production and also contributes to the breakdown of acetylcholine, making it an important target for the development of novel drugs for the treatment of AD [41, 42]. The goal of multi-target new drug therapy is to create novel AD medication candidates with anti-cholinesterase, anti-amyloid-β aggregation, and neuroprotective properties. Enzyme inhibitors had a crucial importance in a variety of disease management situations [43]. The inhibition of AChE and BChE by specific inhibitors is an important therapeutic target for controlling AD, Myasthenia gravis, glaucoma, Lewy body, and dementia [44]. Acetylcholinesterase inhibitors (AChEIs) are utilized in clinical practice treating these problems, as they improve cholinergic function by increasing the amount of ACh in cholinergic synapses [45]. In this study, the 2-aryl-6-carboxamide benzoxazole derivatives (7–48) were investigated in vitro for their ability to inhibit AChE and BChE enzymes. Also, tacrine (TAC) and donepezil were used as positive controls for both cholinergic enzymes. The inhibitory results of the 2-aryl-6-carboxamide benzoxazole derivatives (7–48) are summarized in Table 1.
The novel synthesized compounds effectively inhibited AChE with IC50 values ranging from 12.62 to 67.02 nM and Ki values ranging from 7.74 ± 0.75 to 85.33 ± 11.22 nM. The compound of (2-(1 H-pyrrol-2-yl)benzo[d]oxazol-6-yl)(morpholino)methanone (36) showed the best inhibition profile (Ki: 7.74 ± 0.75 nM) (Table 1). As seen in Table 1, all compounds studied for AChE showed more effective inhibition than TAC (Ki: 95.21 ± 11.87 nM). The CAS binding region and aromatic ring of the benzoxazole group shown in Fig. 3 are changed, and there are also changes in enzyme inhibition.
In compounds 7–12, the AChE inhibition order according to the groups in the CAS binding region is piperidine (Ki: 18.65 ± 2.58 nM) > methyl (Ki: 23.04 ± 1.70 nM) > morpholino (Ki: 28.56 ± 3.96 nM) > ethyl (Ki: 30.60 ± 3.34 nM) > pyrrolidine (Ki: 46.53 ± 6.59) > propyl (Ki: 59.39 ± 3.69). There was not much difference between the ethyl and morpholino groups in terms of inhibition value (Ki: 30.60 ± 3.34 nM for compound 8; Ki: 28.56 ± 3.96 nM for compound 12). The piperidine group showed 3.18 times more effective AChE inhibition than the propyl group. Also, when an additional benzene group is attached to the compounds (7–12) construct, the inhibition order according to the groups in the basic centers: piperidine (Ki: 20.21 ± 1.47 nM) > methyl (Ki: 38.75 ± 2.96 nM) > pyrrolidine (Ki: 41.91 ± 5.64 nM) > morpholino (Ki: 45.73 ± 1.65 nM) > ethyl (Ki: 48.53 ± 9.97 nM) > propyl (Ki: 71.56 ± 12.01 nM).
The piperidine group showed the best inhibition feature in the binding of biphenyl and naphthalene groups to the aromatic ring portion of compounds 7–12 compounds (Ki: 18.37 ± 3.33 nM for compound 17; Ki: 20.21 ± 1.47 nM for compound 23). Compared to the others, a lower inhibition value was observed in the compounds to which the propyl group was attached (Ki: 38.21 ± 0.66 nM for compound 15; Ki: 71.56 ± 12.01 nM for compound 21).
The aromatic ring is pyridine in the structure (compound 25–30) as shown in Fig. 3, the inhibition order according to the groups in the basic centers: morpholino (Ki: 18.32 ± 4.37 nM) > piperidine (Ki: 27.38 ± 2.53 nM) > pyrrolidine (Ki: 47.92 ± 4.70 nM) > propyl (Ki: 50.05 ± 5.13 nM) > ethyl (Ki: 83.13 ± 1.33 nM) > methyl (Ki: 85.33 ± 11.22 nM). The methyl or ethyl group attached to the CAS binding site did not show much change in inhibition (Ki: 85.33 ± 11.22 nM for compound 25; Ki: 83.13 ± 1.33 nM for compound 26). Similarly, being bound to pyrrolidine and propyl groups did not cause much change in inhibition (Ki: 50.05 ± 5.13 nM for compound 27; Ki: 47.92 ± 4.70 nM for compound 28). The morpholino group showed 4.66 times more effective inhibition than the methyl group. When the aromatic ring is the pyrrole ring, inhibition order according to the groups in the basic centers: morpholino (Ki: 7.74 ± 0.75 nM) > piperidine (Ki: 12.31 ± 1.28 nM) > pyrrolidine (Ki: 15.51 ± 1.96 nM) > propyl (Ki: 26.13 ± 3.35 nM) > methyl (Ki: 30.70 ± 2.21 nM) > ethyl (Ki: 47.53 ± 9.80 nM). The morpholino group showed 6.14 times more effective inhibition than the ethyl group. The order of inhibition did not change when the pyridine and pyrrole structure in the aromatic ring shown in Fig. 3 (compounds 37–42 and compound 43–48) were replaced by the furan and thiophene ring. Again, the morpholino group showed the best inhibitor feature among the group.
The novel synthesized 2-aryl-6-carboxamide benzoxazole derivatives (7–48) effectively inhibited BChE with IC50 values ranging from 25.00 to 188.08 nM and Ki values ranging from 20.26 ± 1.56 to 145.13 ± 11.22 nM. As in AChE, compound 2-(1 H-pyrrol-2-yl)benzo[d]oxazol-6-yl)(morpholino)methanone (compound 36) exhibited the best inhibition profile (Ki: 20.26 ± 1.56 nM) against BChE (Fig. 4). As seen in Table 1, except for 8 compounds (Compounds 9, 15, 19, 20, 21, 37, 40, ve 43), the other compounds demonstrated more effective BChE inhibition effects than TAC (Ki: 88.34 ± 9.12 nM). The results showed that the compounds of 7–12 exhibited BChE inhibition order according to the groups in the CAS binding region is morpholino (Ki: 32.33 ± 3.25 nM) > pyrrolidine (Ki: 36.62 ± 10.97 nM) > methyl (Ki: 50.74 ± 6.43 nM) > piperidine (Ki: 74.38 ± 16.89 nM) > ethyl (Ki: 76.95 ± 5.25 nM) > propyl (Ki: 111.23 ± 4.67 nM). There was not much difference between the ethyl and piperidine groups in terms of inhibition value (Ki: 76.95 ± 5.25 nM for compound 8; Ki: 74.38 ± 16.89 nM for compound 11). The morpholino group showed 3.44 times more effective inhibition than the propyl group. When an additional phenyl group is attached to the compound 7–12 constructs, the inhibition order according to the groups in the basic centers: piperidine (Ki: 49.18 ± 11.13 nM) > morpholino (Ki: 56.65 ± 1.57 nM) > methyl (Ki: 65.38 ± 14.05 nM) > ethyl (Ki: 75.25 ± 13.78 nM) > pyrrolidine (Ki: 84.03 ± 12.45 nM) > propyl (Ki: 95.74 ± 7.32 nM). As in AChE, the piperidine aromatic ring showed the most effective inhibition in this group in BChE. Also, when an additional benzene group is linked to the compound 7–12 constructs, inhibition order according to the groups in the basic centers: morpholino (Ki: 50.69 ± 14.02 nM) > piperidine (Ki: 69.37 ± 11.34 nM) > pyrrolidine (Ki: 72.45 ± 5.66 nM) > methyl (Ki: 121.36 ± 15.45 nM) > ethyl (Ki: 136.03 ± 30.69 nM) > propyl (Ki: 145.13 ± 11.22 nM). While the piperidine group was more effective in AChE, the morpholino group was more effective in BChE. The naphthalene group was not effective in BChE inhibition compared to other functional groups.
The aromatic ring is pyridine in the structure (compound 25–30) shown in Fig. 3, the inhibition order according to the groups in the basic centers: morpholino (Ki: 45.35 ± 3.04 nM) > piperidine (Ki: 47.90 ± 8.42 nM) > pyrrolidine (Ki: 52.92 ± 11.67 nM) > ethyl (Ki: 54.39 ± 4.85 nM) > methyl (Ki: 66.40 ± 13.58 nM) > propyl (Ki: 69.69 ± 4.10 nM). The morpholino or piperidine group attached to the CAS binding site did not show much change in inhibition (Ki: 47.90 ± 8.42 nM for compound 29; Ki: 45.35 ± 3.04 nM for compound 30). Similarly, being bound to pyrrolidine and ethyl groups did not cause much change in inhibition (Ki: 54.39 ± 4.85 nM for compound 26; Ki: 52.92 ± 11.67 nM for compound 28). The aromatic ring is pyrrol in the structure the inhibition order according to the groups in the basic centers: morpholino (Ki: 20.26 ± 1.56 nM) > ethyl (Ki: 23.01 ± 1.52 nM) > propyl (Ki: 38.47 ± 8.43 nM) > methyl (Ki: 41.99 ± 6.32 nM) > piperidine (Ki: 44.46 ± 7.43 nM) > pyrrolidine (Ki: 48.22 ± 2.56 nM). The aromatic ring is furan, morpholino (Ki: 48.56 ± 4.55 nM) > ethyl (Ki: 59.44 ± 8.01 nM) > propyl (Ki: 65.24 ± 8.33 nM) > piperidine (Ki: 75.48 ± 18.45 nM) > pyrrolidine (Ki: 95.64 ± 20.22 nM) > methyl (Ki: 123.34 ± 15.34 nM). The aromatic ring is thiophene, morpholino (Ki: 22.67 ± 3.07 nM) > piperidine (Ki: 46.56 ± 2.10 nM) > ethyl (Ki: 61.02 ± 11.13 nM) > propyl (Ki: 72.45 ± 12.56 nM) > pyrrolidine (Ki: 87.55 ± 13.56 nM) > methyl (Ki: 120.16 ± 25.67 nM). As in AChE, while the morpholino group shows the best inhibition, the order of inhibition differs. When the Selectivity index (AChE/BChE) is evaluated, it is seen that the highest Selectivity index (AChE/BChE; 2.07) value was found for the N,N-diethyl-2-(1H-pyrrol-2-yl)benzo[d]oxazole-6-carboxamide (compound 32).
In summary, the 42 novel benzoxazole-based derivatives presented here were found to have various inhibitory potentials and selectivity index against AChE and BChE enzymes. Changing the inhibitory effect and selectivity index with small differences in the molecule allows compounds to target the relevant enzyme with a comprehensive structure-activity relationship. It is known that in late-stage Alzheimer’s dementia (AD), the AChE level in the brain gradually decreases, but BuChE activity remains the same or can increase up to 165% of the normal level [46]. Therefore, BuChE is proposed as a proposed drug target for the treatment of late-stage AD, and it has also been reported that inhibitors with more bulky moieties can increase the BChE selectivity index [47]. In this context, compound 32 exhibits approximately 5.5 times higher BChE selectivity compared to compound 36. This can be explained by the presence of a slightly bulky N-N-diethyl group in compound 32 compared to the morpholine group found in compound 36. Lastly, the binding affinities of the compounds towards the active sites of AChE and BChe enzymes were investigated by molecular docking and dynamic simulations.
Molecular docking and molecular dynamics simulations
Molecular modeling and molecular dynamics simulations are critical in the development of novel and efficient cholinesterase inhibitors. The active areas of cholinesterase enzymes contain critical amino acids that could be used in the development of effective drugs. Tyr70, Asp72, Tyr121, Trp279, and Tyr334 are found in the active site of AChE, which is 20 Å deep and 5 wide. Furthermore, the active site of the enzyme, CAS, is deeper. CAS is made up of the catalytic triad His440, Glu327, and Ser200, as well as the essential amino acid Trp84. BChE’s active site is also about 20 deep and slightly wider than AChE’s. The active site contains the catalytic triad of amino acids Ser198, His438, and Glu325. Glu197, Trp231, Phe329 (acyl-binding pocket), Asp70, and Tyr332 are also found in the active site (PAS) [48]. Compounds must interact with these residues to inhibit choline esterase, and this interaction must last for a certain amount of time [49, 50].
Compound 36 in complex with AChE
In molecular modeling studies, it was aimed determine the binding modes of compounds to the active sites of AChE. Modeling studies were performed with compounds 7–48, tacrine, and donepezil (co-crystallized ligand in 1EVE). In modeling studies, the proposed binding modes of ligands were inserted into the active gorge of AChE.
The docking scores of the synthesized compounds were calculated as -6.075 and − 7.291 kcal/mol (Table 2). The docking scores of the compounds are proportional to the order of the experimental IC50 values. Compound 36 with the highest docking score (-7.291 kcal/mol) has the best IC50 (12.62 nM) value, which also supports this situation.
Overall, we found that the binding modes for all compounds (7–48) were similar pose present in AChE (Fig. 5A). In addition, when donepezil and compound 36 were superimposed on the active site of the protein, it was observed that their exposures were close (Fig. 5B). The docking scores of the most active compound 36 (IC50: 12.62 nM) and reference compound donepezil (IC50: 69.30 nM) against AChE were calculated as -7.291 and − 6.492 kcal/mol, respectively. As a result of modeling studies of compound 36 against AChE, its interaction with the active site of the protein was investigated.
Compound 36 formed π-π and π-cation interactions with His440, located in the CAS region of the protein, and π-π interaction with Trp84, with the pyrrole ring in the second position. Compound 36 has formed π-π interactions with Phe330 and Tyr334 via the benzoxazole ring and also has hydrophobic interactions with Tyr70, Trp279, and Tyr121 located in the PAS region of the protein (Fig. 6A). The results show that the compound interacts with residues in both PAS and CAS. In the docking study with donepezil, donepezil formed π-cation interactions with Trp84, Phe330, and Tyr334 and formed π-π interactions with Trp84 and Trp279. It can be said that compound 36 has interactions like donepezil in the active gorge of AChE (Fig. 6B). Unlike donepezil, the interaction of compound 36 with one of the important key residues, Trp84, is thought to cause both the IC50 and docking score of the compound to be higher than donepezil.
Molecular dynamics simulations were performed for 100 ns with the selected pose of Compound 36 in the AChE complex. Throughout the simulation, the average RMSD values of compound 36 and protein were calculated as 1.98 Å and 1.28 Å, respectively (Fig. 7). The RMSD plot shows that compound 36 localizes to the active site of the protein and remains stable. The results of this simulation indicate that compound 36 binds strongly to the active site of AChE, suggesting that it possesses the lowest IC50.
When the interaction of compound 36 with the protein was examined by the simulation, it was observed that it had significant hydrophobic interactions, especially with Tyr70, Trp84, Phe331, Phe330, Tyr334, Trp279, Tyr121, and His440 (Fig. S127). When the time graph of the interaction is examined (the timeline representation of the interactions and contacts), it can be said that compound 36 interacts with Trp84, Phe331, and Tyr334 roughly throughout the simulation (Fig. S128).
Compound 36 in complex with BChE
The synthesized compounds 7–48, tacrine, and donepezil were docked at the active site of BChE, and binding poses and interactions of the ligands were investigated. The binding poses of compounds 7–48 were found to be similar. Donepezil and compound 36 were superimposed on the active site of the protein, and their binding poses were observed (Fig. 8). As a result of molecular modeling studies, the docking scores of the compounds (7–48) were calculated between − 2.549 and − 6.719 kcal/mol (Table 2).
Regarding docking studies, the docking scores of the most active compound 36 (IC50: 25.45 nM) and reference compound donepezil (IC50: 63.00 nM) against BChE were calculated as -6.719 and − 5.818 kcal/mol, respectively. Compound 36 formed hydrogen bond with Asp70 (distance: 2.12 Å) and formed π-π interactions with Tyr332 (PAS) in the active site of BChE via the pyrrole ring. In addition, it interacted hydrophobicically with Phe329 (acyl-binding site) and Trp231 (Fig. 9). When the interaction of donepezil with the active site of BChE is examined, donepezil formed pi-cation with Tyr332 via the piperidine ring and formed a salt bridge with Asp70. Donepezil also had hydrophobic interactions with Phe329 and Trp231, which are important residues of the binding site. Compound 36 and donepezil were found to have similar interactions at the active site of BChE. It can be said that the hydrogen bonding of compound 36 with Asp70 has a significant effect on binding to the active site of the protein more strongly than donepezil.
3D mode of interactions of compound 36 (A) and donepezil (B) with receptor BChE. C atoms are presented in green; N atoms are presented in blue and O atoms are presented in red respectively; hydrogen bonds are shown in yellow, π-cation interactions are shown in dark green; π-π interactions are shown in light blue
Molecular dynamic simulations were carried out for 100ns with the selected pose of compound 36 with the best docking score. The RMSD value of the ligand until the 15th ns is between 2.4 Å and 3.2 Å, while the RMSD value of the ligand from the 20th ns until the end of the stimulation is between 4.8 Å and 7.0 Å (Fig. 10).
It is thought that this change in RMSD values is caused by compound 36 interacting with Glu197 at the beginning of the simulation, closing to Asp70, and beginning to interact with Asp70 due to the change in its conformation from about 15 ns (Fig. S129). It can be said that after the 15th ns, the RMSD value for ligand reached roughly the plateau value. During the simulation, the average RMSD value of the protein is 0.4 Å. During the simulation, compound 36 interacted with Asp70, Trp82, Trp231, Phe239, Leu285, and Tyr332 (Fig. S130).
In summary, the 42 synthesized compounds demonstrate a targeted interaction with the active sites of both AChE and BChE enzymes, mimicking the binding position of donepezil and focusing on the CAS and PAS domains of both enzymes. Notably, compound 36, identified as the most potent inhibitor in in vitro enzyme inhibition studies, exhibited a superior binding affinity for both enzymes compared to donepezil and other compounds, as indicated by docking scores.
The heightened inhibition potential of compound 36, surpassing that of donepezil, can be attributed to the pyrrole structure establishing a π-π interaction with Trp84, a crucial residue in the AChE active site. This enhanced interaction is further supported by the extended engagement of compound 36 with Trp84 observed in molecular dynamics simulations. Additionally, the pyrrole N-H group in compound 36 displays a significantly stronger binding affinity than donepezil, forming a hydrogen-bonding interaction with Asp70 in the BChE active site. Molecular dynamics studies further illustrate a long-range interaction between compound 36 and Asp70, emphasizing the importance of this residue in the compound’s binding mechanism.
ADME study
Prediction of the blood-brain barrier (BBB) is useful for drugs targeting the central nervous system (CNS) to have a notion of a compound’s ability to permeate this barrier. Therefore, QPlogBB was used to describe the properties of cholinesterase inhibitors. ADME studies also include estimated central nervous system (CNS) activities, blood/brain partition coefficients, oral percent absorption, cell permeability (MDCK cell permeability), and BBB permeation (Table S1). The majority of the estimated parameters are within acceptable ranges according to the reported guidelines.
All of the compounds (except for compound 30) have the potential to be active in the CNS. The QlogBB coefficients of the compounds range from − 0.568 to 0.116, with these coefficients in the recommended range (–3.0–1.2). The predicted apparent MDCK cell permeability (QPPMDCK) values of all compounds are within acceptable limits. All but compounds 15, 21, 45, and 48 were found to be BBB permeable. Also, compounds generally obey Lipinski’s rule of five. The percent absorption values of its compounds are between 92.55 and 100. These findings inform us that the compounds may be promising cholinesterase inhibitor candidates.
Conclusion
In conclusion, we have successfully introduced, for the first time, a new class of compounds — 2-aryl-6-carboxamide benzoxazole derivatives — that exhibit inhibitory effects on cholinesterases. Enzyme assays and kinetics studies were employed to evaluate the inhibitory effects of 42 newly synthesized compounds on both AChE and BChE.
This study holds the potential to provide preliminary data for the development of a design strategy in medicinal chemistry research, contributing to the chemical library for compounds targeting the CAS and PAS binding sites of cholinesterases.
Promisingly, all designed compounds demonstrated higher inhibitory effects on AChE (IC50: 12.62 nM − 67.02 nM) compared to donepezil and tacrine (IC50: 69.30 nM, 105.21). Specifically, compounds 10–12, 17, 28–36, 42, and 48 (IC50: 25.45 nM − 61.25 nM) exhibited greater effectiveness against BChE than donepezil and tacrine (IC50: 63.00 nM, 94.13 nM). The most potent compound among the designed molecules, compound 36, demonstrated inhibition concentrations of 12.62 nM for AChE and 25.45 nM for BChE. In molecular docking studies, compound 36 exhibited higher binding affinity for both AChE (-7.29 kcal/mol) and BChE (-6.71 kcal/mol) compared to donepezil (-6.49 kcal/mol and − 5.057 kcal/mol, respectively). Molecular docking studies revealed that compound 36 binds to both the CAS and PAS domains of proteins, with the pyrrole ring in its structure binding to the CAS region and the benzoxazole ring playing a crucial role in binding to the PAS region.
Molecular dynamics (MD) simulations were conducted to assess the stability of compound 36 in the active sites of target proteins. The results indicated that compound 36 remained stable in the active gorges of both AChE (average RMSD: 1.98 Å) and BChE (average RMSD: 2.2 Å). Furthermore, the presented compounds exhibited suitable ADME properties and druggability potential.
Cholinesterase (ChE) inhibitors, frequently employed in AD treatment, target AChE and BChE. The compounds designed in our study demonstrated significant inhibition effects on cholinesterases, suggesting potential use in the treatment of AD, a prevalent and global disease. However, the effects of these compounds on AD require confirmation through further experimental verification.
Materials and methods
Commercially available materials were used without further purification. 4-Amino-3-hydroxy-benzoic acid (Acros), benzaldehyde (Merck), biphenyl-4-carboxaldehyde (Sigma-Aldrich) α-naphthaldehyde (Merck), 4-pyridinecarboxaldehyde (Merck), 2-pyrrolecarboxaldehyde (Saffchemical) Furfural (Sigma-Aldrich), 2-thiophenecarboxaldehyde (Sigma-Aldrich), dimethylamine hydrochloride (Acros), diethylamine hydrochloride (Acros), diisopropylamine (Acros), pyrrolidine (Acros) and piperidine (Acros) and morpholine (Acros) were used as synthesis starting materials. As solvent, catalyst and co-reactant; methanol (Isolab), ethanol (Isolab), sulfuric acid (H2SO4; 98%, Isolab), dichloromethane (DCM, Sigma-Aldrich), N’N’-dimethylformamide (DMF, Merck), sodium cyanide (NaCN, Sigma-Aldrich), aluminum trichloride (AlCl3, Merck), n-hexane (Abcr), ethylacetate (Abcr), magnesium sulfate (MgSO4, Isolab), sodium bicarbonate (NaHCO3, Sigma-Aldrich) were used. In addition, Kieselgel 60 F254 2 mm thick coated ready-made aluminum plates (Silicycle) were used in thin layer chromatography (TLC) studies. 1H-NMR, and 13C-NMR, spectra were recorded at 400, and 100 MHz, respectively, on a Varian-Agilent 400 MHz instrument using Me4Si as an internal standard. All column chromatography was performed on silica gel (60 mesh, Silycycle). New compounds for HRMS were tested on a Thermo Scientific Q Exactive MS/MS system ESI spectrometer. Chemical shift multiplicities are represented as follows: (s = singlet, d = doublet, t = triple, dd = double doublet, and m = multiplet).
Chemistry
Synthesis of methyl 4-amino-3-hydroxybenzoate
The 4-amino-3-hydroxy-benzoic acid (5 mmol) was dissolved in 10 mL of methanol and refluxed for 12 h under the catalyst of a few drops (5% mol) of concentrated H2SO4. After the completion of the reaction was checked with TLC, the reaction was finished and cooled to room temperature. It was neutralized with 50 mL of water and 1 N NaHCO3 until pH:7.5. The resulting aqueous phase was extracted with 3 × 15 mL of ethyl acetate. The organic phase was separated, the solvent was evaporated and the crude product was purified by column chromatography in the appropriate n-hexane/ethylacetate (5/1) mobile phase (Scheme 1) [51].
General procedure for the synthesis 2-aryl benzoxazole derivatives
A mixture of methyl 4-amino-3-hydroxybenzoate (1 mmol) was dissolved in 5 mL ethanol and the corresponding aromatic carboxaldehyde derivatives (1.1 mmol) were added to the reaction mixture. The reaction mixture was stirred for a period of 6–24 h at room temperature and the reaction completion was controlled with the TLC method. After observing that the starting materials were disappeared, the reaction mixture was poured into ice water to get the precipitate. The resulting precipitate was filtered off and washed with cold water. The solid products were used in the second step without isolation. Solid products (1 mmol) were dissolved in 5 mL of DMF and a catalytic amount of NaCN was added. The reaction mixture was stirred at room temperature for 1 h. With the starting material disappearing, the reaction mixture was transferred to the ice-water mixture to get the precipitate. The resulting precipitate was filtered off and washed with brine. The final products were purified with column chromatography 5:1 (n-hexane: ethylacetate) (Scheme 1) [52].
General procedure for the synthesis 2-aryl-6-carboxamide benzoxazole derivatives
2-Aryl benzoxazole derivatives (1 mmol) synthesized in the previous step were dissolved in 4 mL of dichloromethane (DCM) and 1.2 mmol of cyclic or linear aliphatic secondary amine (pyrrolidine, piperidine, morpholine or dimethylamine, diethylamine, dipropylamine) derivatives were added. After the reaction mixture was stirred at room temperature for 10 min, a catalytic amount of aluminum (III) chloride (AlCl3) was added. The reaction mixture was stirred at room temperature for 24 h. The completion of the reaction was checked with TLC and the reaction was finished by the addition of water. The resulting mixture was extracted by adding 3 × 15 mL of dichloromethane (DCM) and 50 mL of water. The organic phases were collected, dried with MgSO4 and filtered. The crude product obtained by evaporation of the organic solvent was washed successively with diethyl ether and cyclohexane.
In fact, the reaction conditions were first adjusted in dichloromethane (DCM) at room temperature by addition of straight chain or cyclic secondary amines [53]. However, the reaction did not proceed at this temperature and the situation did not change with the increase in temperature. Therefore, in order to increase the reactivity of the ester carbonyl, AlCl3 was added to the reaction mixture as a catalytic amount of Lewis acid. The mechanism of the mentioned reaction is presented in Scheme 1.
N,N -dimethyl-2-phenylbenzo[d]oxazole-6-carboxamide (7)
White solid, m.p. 111–112 °C, Rf; 0.65 in ethylacetate/n-hexane (3/1), Yield; 74%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.22–8.18 (m, 2 H, Ar-H), 7.72 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.64 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.51–7.46 (m, 3 H, Ar-H), 7.39 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 3.09 (bs, 3 H, -CH3), 2.98 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.8, 164.3, 150.3, 143.1, 133.2, 131.9, 128.9, 127.7, 126.7, 123.9, 119.7, 110.0, 39.7, 35.5. HRMS [M + H] (C16H15N2O2): Calculated: 267.1134; Found: 267.1167.
N,N-diethyl-2-phenylbenzo[d]oxazole-6-carboxamide (8)
Gray solid, m.p. 129–130 °C, Rf; 0.74 in ethylacetate/n-hexane (3/1), Yield; 71%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.25–8.21 (m, 2 H, Ar-H), 7.75 (dd, J = 0.5 Hz, J = 8.1 Hz, 1H, Ar-H), 7.61 (dd, J = 0.5 Hz, J = 1.5 Hz, 1H, Ar-H), 7.54–7.48 (m, 3 H, Ar-H), 7.36 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 3.54 (bs, 2 H, -CH2-), 3.29 (bs, 2 H, -CH2-), 1.33–1.05 (m, 6 H, -CH3(2x)). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.5, 164.2, 150.4, 142.8, 134.2, 131.8, 128.9, 127.7, 126.8, 123.2, 119.9, 109.1, 43.4, 39.5, 14.1, 13.0. HRMS [M + H] (C18H19N2O2): Calculated: 295.1447; Found: 295.1153.
2-Phenyl-N,Ndipropylbenzo[d]oxazole-6-carboxamide (9
Orange viscous liquid, Rf; 0.65 in ethylacetate/n-hexane (2/1), Yield; 95%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.27–8.23 (m, 2 H, Ar-H), 7.76 (dd, J = 0.60 Hz, J = 8.13 Hz, 1H, Ar-H), 7.61 (dd, J = 0.60 Hz, J = 1.47 Hz, 1H, Ar-H), 7.55–7.50 (m, 3 H, Ar-H), 7.35 (dd, J = 1.47 Hz, J = 8.13 Hz, 1H, Ar-H), 3.48 (bs, 2 H, -CH2-), 3.21 (bs, 2 H, -CH2-), 1.71 (bs, 2 H, -CH2-), 1.56 (bs, 3 H, -CH2-), 0.99 (bs, 2 H, -CH3), 0.74 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.0, 164.2, 150.4, 142.7, 134.4, 131.8, 129.0, 127.7, 126.8, 123.3, 119.9, 109.3, 50.9, 46.5, 21.9, 20.7, 11.4, 11.1. HRMS [M + H] (C20H23N2O2): Calculated: 323.1760; Found: 323.1784.
(2-Phenylbenzo[d oxazol-6-yl)(pyrrolidin-1-yl)methanone (10)
White solid, m.p. 144–145 °C, Rf; 0.68 in ethylacetate/n-hexane (1/1), Yield; 80%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.29–8.25 (m, 2 H, Ar- H), 7.80–7.75 (m, 2 H, Ar-H), 7.58–7.51 (m, 4 H, Ar-H), 3.69 (t, J = 7.0 Hz, 2 H, -CH2-), 3.50 (t, J = 6.6 Hz, 2 H, 2 H, -CH2-), 2.03–1.96 (m, 2 H, 2 H, -CH2-), 1.93–1.87 (m, 2 H, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.0, 164.5, 150.3, 143.3, 134.2, 131.9, 129.0, 127.8, 126.8, 124.0, 119.6, 110.0, 49.9, 46.4, 26.5, 24.5. HRMS [M + H] (C18H17N2O2): Calculated: 293.1290; Found: 293.1324.
(2-Phenylbenzo[d oxazol-6-yl)(piperidin-1-yl)methanone (11)
Light orange solid, m.p. 127–128 °C, Rf; 0.80 in ethylacetate/n-hexane (2/1), Yield; 65%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.29–8.24 (m, 2 H, Ar-H), 7.77 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.66 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.57–7.53 (m, 3 H, Ar-H), 7.40 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 3.74 (bs, 2 H, -CH2-), 3.40 (bs, 2 H, -CH2-), 1.70 (bs, 4 H, -CH2-), 1.56 (bs, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 164.3, 150.4, 143.0, 133.4, 131.9, 129.0, 127.8, 126.8, 123.7, 119.9, 109.7, 48.9, 43.4, 26.7, 25.8, 24.6. HRMS [M + H] (C19H19N2O2): Calculated: 307.1447; Found: 307.1480.
Morpholino(2-phenylbenzo[d oxazol-6-yl)methanone (12)
Light brown solid, m.p. 117–118 °C, Rf; 0.70 in ethylacetate/n-hexane (2/1), Yield; 74%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.28–2.24 (m, 2 H, Ar-H), 7.79 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.68 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.57–7.52 (m, 3 H, Ar-H), 7.41 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 3.86–3.50 (m, 8 H). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 169.8, 164.6, 150.5, 143.5, 132.1, 132.0, 129.0, 127.8, 126.7, 123.9, 120.0, 110.1, 66.9. HRMS [M + H] (C18H17N2O3): Calculated: 309.1239; Found: 309.1253.
2-([1,1’-Biphenyl]-4-yl)-N,N-dimethylbenzo[d]oxazole-6-carboxamide (13)
Dark orange solid, m.p. 185–186°C, Rf; 0.84 in ethylacetate/n-hexane (3/1), Yield; 70%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.36–8.31 (m, AA’BB’ system, 2 H, Ar-H), 7.81–7.76 (m, 3 H, Ar-H), 7.76–7.70 (m, 3 H, Ar-H), 7.52–7.47 (m, 2 H, Ar-H), 7.44 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.43–7.38 (m, 1H, Ar-H), 3.16 (bs, 3 H, -CH3), 3.06 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170, 9, 164.3, 150.4, 144.6, 143.2, 139.9, 133.2, 129.0, 128.3, 128.2, 127.6, 127.2, 125.5, 124.0, 119.7, 110.0, 39.8, 35.6. HRMS [M + H] (C22H19N2O2): Calculated: 343.1447; Found: 343.1481.
2-([1,1’-Biphenyl]-4-yl)-N,N-diethylbenzo[doxazole-6-carboxamide (14)
Dark orange viscous liquid, Rf; 0.70 in ethylacetate/n-hexane (3/1), Yield; 66%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.37–8.29 (m, AA’BB’ system, 2 H, Ar-H), 7.80–7.76 (m, 3 H, Ar-H), 7.70–7.64 (m, 3 H, Ar-H), 7.52–7.46 (m, 2 H, Ar-H), 7.43–7.37 (m, 2 H, Ar-H),3.58 (bs, 2 H, -CH2-), 3.33 (bs, 2 H, -CH2-), 1.28–1.14 (m, 6 H, -CH3(2x)). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.6, 164.1, 150.4, 144.6, 142.9, 139.9, 134.2, 129.0, 128.2, 128.1, 127.6, 127.2, 125.6, 123.2, 119.9, 109.2, 43.6, 39.5, 14.1, 13.0. HRMS [M + H] (C24H23N2O2): Calculated: 371.1760; Found: 371.1781.
2-([1,1’-Biphenyl]-4-yl)-N,N-dipropylbenzo[d]oxazole-6-carboxamide (15)
Light yellow solid, m.p. 87–88°C, Rf; 0.85 in ethylacetate/n-hexane (1/1), Yield; 85%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.35–8.32 (m, AA’BB’ system, 2 H, Ar-H), 7.80–7.76 (m, 3 H, Ar-H), 7.70–7.65 (m, 2 H, Ar-H), 7.63 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.52–7.46 (m, 2 H, Ar-H), 7.44–7.39 (m, 1H, Ar-H), 7.37 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 3.50 (bs, 2 H, -CH2-), 3.23 (bs, 2 H, -CH2-), 1.7. (bs, 2 H, -CH2-), 1.56 (bs, 2 H, -CH2-), 1.01 (bs, 3 H, -CH3), 0.76 (bs, 2 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.0, 164.1, 150.4, 144.6, 142.8, 139.9, 134.3, 129.0, 128.2, 128.1, 127.6, 127.2, 125.6, 123.4, 119.9, 109.3, 50.9, 46.5, 20.7, 20.3, 11.5, 11.1. HRMS [M + H] (C26H27N2O2): Calculated: 399.2073; Found: 399.2084.
(2-([1,1’-Biphenyl]-4-yl)benzo[d]oxazol-6-yl)(pyrrolidin-1-yl)methanone (16)
White solid, m.p. 159–160°C, Rf; 0.86 in ethylacetate/n-hexane (3/1), Yield; 61%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.37–8.31 (m, AA’BB’ system, 2 H, Ar-H), 7.82–7.74 (m, 4 H, Ar-H), 7.70–7.66 (m, 2 H, Ar-H), 7.56 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.52–7.47 (m, 2 H, Ar-H), 7.44–7.38 (m, 1H, Ar-H), 3.70 (t, J = 6.9 Hz, 2 H, -CH2-), 3.51 (t, J = 6.6 Hz, 2 H, -CH2-), 2.03–1.97 (m, 2 H, -CH2-), 1.94–1.88 (m, 2 H, -CH2). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.0, 164.4, 150.3, 144.6, 143.4, 139.9, 134.2, 129.0, 128.3, 128.2, 127.6, 127.2, 125.5, 124.1, 119.6, 110.0, 49.9, 46.4, 26.5, 24.5. HRMS [M + H] (C24H21N2O2): Calculated: 369.1603; Found: 369.1633.
(2-([1,1’-Biphenyl]-4-yl)benzo[d]oxazol-6-yl)(piperidin-1-yl)methanone (17)
White solid, m.p. 165–166°C, Rf; 0.82 in ethylacetate/n-hexane (2/1), Yield; 88%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.35–8.32 (m, AA’BB’ system, 2 H, Ar-H), 7.80–7.76 (m, 3 H, Ar-H), 7.70–7.65 (m, 3 H, Ar-H), 7.52–7.47 (m, 2 H, Ar-H), 7.44–7.38 (m, 2 H, Ar-H), 3.74 (bs, 2 H, -CH2-), 3.42 (bs, 2 H, -CH2-), 1.76–1.66 (m, 4 H, -CH2- (x2)), 1.61–1.57 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 164.2, 150.5, 144.6, 143.1, 139.9, 133.4, 129.0, 128.2, 128.1, 127.6, 127.2, 125.5, 123.7, 119.8, 109.7, 49.0, 42.1, 30.9, 24.6. HRMS [M + H] (C25H23N2O2): Calculated: 383.1760; Found: 383.1769.
(2-([1,1’-Biphenyl]-4-yl)benzo[d]oxazol-6-yl)(morpholino)methanone (18)
Light yellow solid, m.p. 180–181°C, Rf; 0.90 in ethylacetate/n-hexane (3/1), Yield; 79%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.36–8.32 (m, AA’BB’ system, 2 H, Ar-H), 7.82–7.77 (m, 3 H, Ar-H), 7.70 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.69–7.66 (m, 2 H, Ar-H), 7.52–7.47 (m, 2 H, Ar-H), 7.45–7.41 (m, 2 H, Ar-H), 3.85–3.55 (m, 8 H, -CH2- (x4)). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.8, 164.5, 150.5, 144.8, 143.6, 139.8, 132.1, 129.0, 128.3, 128.2, 127.7, 127.2, 125.4, 123.9, 120.0, 110.1, 66.9. HRMS [M + H] (C24H21N2O3): Calculated: 385.1552; Found: 385.1567.
N,Ndimethyl-2-(naphthalen-1-yl)benzo[d]oxazole-6-carboxamide (19)
Light orange solid, m.p. 134–136 °C, Rf; 0.76 in ethylacetate/n-hexane (2/1), Yield; 81%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.46 (d, J = 8.7 Hz, 1H, Ar-H), 8.43 (dd, J = 1.2 Hz, J = 7.3 Hz, 1H, Ar-H), 8.03 (d, J = 8.2 Hz, 1H, Ar-H), 7.92 (d, J = 8.2 Hz, 1H, Ar-H), 7.87 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.73 (m, 2 H, Ar-H), 7.61–7.56 (m, 2 H, Ar-H), 7.46 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 3.15 (bs, 3 H, -CH3), 3.04 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.0, 164.2, 149.7, 143.3, 133.9, 133.4, 132.7, 130.6, 129.6, 128.7, 128.0, 126.5, 126.2, 124.9, 123.9, 123.1, 120.0, 109.9, 39.8, 35.6. HRMS [M + H] (C20H17N2O2): Calculated: 317.1290; Found: 317.1277.
N,Ndiethyl-2-(naphthalen-1-yl)benzo[d]oxazole-6-carboxamide (20)
Dark orange viscous liquid, Rf; 0.92 in ethylacetate/n-hexane (3/1), Yield; 63%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.47 (dd, J = 0.7 Hz, J = 8.4 Hz, 1H, Ar-H), 8.44 (dd, J = 1.3 Hz, J = 7.3 Hz, 1H, Ar-H), 8.05 (d, J = 8.4 Hz, 1H, Ar-H), 7.94 (d, J = 8.4 Hz, 1H, Ar-H), 7.88 (dd, J = 0.7 Hz, J = 8.4 Hz, 1H, Ar-H), 7.74–7.68 (m, 2 H, Ar-H), 7.64–7.56 (m, 2 H, Ar-H), 7.42 (dd, J = 1.3 Hz, J = 8.4 Hz, 1H, Ar-H), 3.57 (bs, 2 H, -CH2-), 3.34 (bs, 2 H, -CH2-), 1.35 (bs, 3 H, -CH3), 1.12 (bs, 3 H, -CH3).13C-NMR (100 MHz, CDCl3) ppm δ = 170.6, 164.0, 149.8, 143.0, 134.4, 134.0, 132.7, 130.6, 129.5, 128.7, 128.0, 126.5, 126.2, 124.9, 123.2, 123.1, 120.2, 109.0, 43.4, 39.5, 14.1, 13.0. HRMS [M + H] (C22H21N2O2): Calculated: 345.1603; Found: 345.1625.
2-(Naphthalen-1-yl)-N,Ndipropylbenzo[d]oxazole-6-carboxamide (21)
Light yellow viscous liquid, Rf; 0.84 in ethylacetate/n-hexane (1/1), Yield; 91%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.79 (s, 1H, Ar-H), 8.31 (dd, J = 1.7 Hz, J = 8.6 Hz, 1H, Ar-H), 8.02–7.97 (m, 2 H, Ar-H), 7.93–7.88 (m, 1H, Ar-H), 7.81 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.67–7.65 (m, 1H, Ar-H), 7.62–7.56 (m, 2 H, Ar-H), 7.38 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 3.50 (bs, 2 H, -CH2-), 3.23 (bs, 2 H, -CH2-), 1.73 (bs, 2 H, -CH2-), 1.57 (bs, 2 H, -CH2-), 1.01 (bs, 3 H, -CH3), 0.76 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.1, 164.4, 150.5, 142.8, 134.9, 134.4, 132.9, 129.0, 128.9, 128.4, 128.0, 127.9, 127.0,0 124.0, 123.9, 123.4, 119.9, 109.3, 50.9, 46.5, 21.9, 20.7, 11.4, 11.1. HRMS [M + H] (C24H25N2O2): Calculated: 373.1916; Found: 373.1925.
(2-(Naphthalen-1-yl)benzo[doxazol-6-yl)(pyrrolidin-1-yl)methanone (22)
Light brown solid, m.p. 165–166 °C, Rf; 0.90 in ethylacetate/n-hexane (4/1), Yield; 56%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.47 (dd, J = 1.0 Hz, J = 7.9 Hz, 1H, Ar-H), 8.46 (dd, J = 1.0 Hz, J = 7.9 Hz, 1H, Ar-H), 8.06 (d, J = 8.2 Hz, 1H, Ar-H), 7.95 (d, J = 8.2 Hz, 1H, Ar-H), 7.88 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.84 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.75–7.70 (m, 1H, Ar-H), 7.64–7.58 (m, 3 H, Ar-H), 3.71 (t, J = 6.8 Hz, 2 H, -CH2-), 3.52 (t, J = 6.8 Hz, 2 H, -CH2-), 2.04–1.98 (m, 2 H, -CH2-), 1.95–1.88 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.0, 164.3, 149.7, 143.5, 134.4, 134.0, 132.7, 130.7, 129.6, 128.7, 128.1, 126.5, 126.2, 124.9, 123.9, 123.2, 119.9, 109.9, 49.9, 46.4, 26.5, 24.5. HRMS [M + H] (C22H19N2O2): Calculated: 343.1447; Found: 343.1437.
(2-(Naphthalen-1-yl)benzo[doxazol-6-yl)(piperidin-1-yl)methanone (23)
Light yellow solid, m.p. 158–159 °C, Rf; 0.70 in ethylacetate/n-hexane (1/1), Yield; 68%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.47 (d, J = 8.7 Hz, 1H, Ar-H), 8.46 (dd, J = 1.2 Hz, J = 7.4 Hz, 1H, Ar-H), 8.06 (d, J = 8.2 Hz, 1H, Ar-H), 7.95 (d, J = 8.2 Hz, 1H, Ar-H), 7.88 (d, J = 8.2 Hz, 1H, Ar-H), 7.75–7.69 (m, 2 H, Ar-H), 7.65–7.58 (m, 2 H, Ar-H), 7.45 (dd, J = 1.2 Hz, J = 8.2 Hz, 1H, Ar-H), 3.76 (bs, 2 H, -CH2-), 3.44 (bs, 2 H, -CH2-), 1.76–1.67 (m, 4 H, -CH2- (x2)), 1.62–1.52 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 164.1, 149.8, 143.2, 134.0, 133.6, 132.7, 130.7, 129.6, 128.7, 128.0, 126.5, 126.2, 124.9, 123.6, 123.2, 120.1, 109.6, 49.9, 43.5, 26.9, 25.6. HRMS [M + H] (C23H21N2O2): Calculated: 357.1603; Found: 357.1611.
Morpholino(2-(naphthalen-1-yl)benzo[d]oxazol-6-yl)methanone (24)
Light gray solid, m.p. 153–154 °C, Rf; 0.80 in ethylacetate/n-hexane (1/1), Yield; 70%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.47 (d, J = 8.6 Hz, 1H, Ar-H), 8.46 (d, J = 7.4 Hz, 1H, Ar-H), 8.07 (d, J = 8.1 Hz, 1H, Ar-H), 7.96 (d, J = 7.5 Hz, 1H, Ar-H), 7.90 (d, J = 8.1 Hz, 1H, Ar-H), 7.76–7.70 (m, 2 H, Ar-H), 7.66–7.58 (m, 2 H, Ar-H), 7.48–7.45 (m, 1H, Ar-H), 3.87–3.54 (m, 8 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.9, 164.4, 149.8, 143.6, 134.0, 132.9, 132.3, 130.6, 129.7, 128.8, 128.1, 126.6, 126.1, 124.9, 123.8, 123.0, 120.3, 110.0, 66.9, 65.8. HRMS [M + H] (C22H19N2O3): Calculated: 359.1396; Found: 359.1415.
N,Ndimethyl-2-(pyridin-4-yl)benzo[d]oxazole-6-carboxamide (25)
White solid, m.p. 157–158 °C, Rf; 0.90 in ethylacetate/n-hexane (3/1), Yield; 96%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.81 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 8.06 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 7.81 (dd, J = 8.2 Hz, 1H, Ar-H), 7.70 (d, J = 1.2 Hz, 1H, Ar-H), 7.45 (dd, J = 1.2 Hz, J = 8.2 Hz, 1H, Ar-H), 3.13 (bs, 3 H, -CH3), 3.01 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.5, 161.9, 150.8, 150.4, 142.6, 134.5, 133.9, 124.4, 121.0, 120.5, 110.3, 39.7, 35.6. HRMS [M + H] (C15H14N3O2): Calculated: 268.1086; Found: 268.1104.
N,Ndiethyl-2-(pyridin-4-yl)benzo[d]oxazole-6-carboxamide (26)
Gray solid, m.p. 110–111 °C, Rf; 0.85 in ethylacetate/n-hexane (1/1), Yield; 84%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.81 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 8.06 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 7.81 (d, J = 8.2 Hz, 1H, Ar-H), 7.65 (d, J = 1.2 Hz, 1H, Ar-H), 7.40 (dd, J = 1.2 Hz, J = 8.2 Hz, 1H, Ar-H), 3.56 (bs, 2 H, -CH2-), 3.28 (bs, 2 H, -CH2-), 1.25 (bs, 3 H, -CH3), 1.13 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.1, 161.8, 150.8, 150.5, 142.3, 135.5, 133.9, 123.7, 121.0, 120.7, 109.5, 43.4, 39.5, 14.1, 12.9. HRMS [M + H] (C17H18N3O2): Calculated: 296.1399; Found: 296.1413.
N,Ndipropyl-2-(pyridin-4-yl)benzo[d]oxazole-6-carboxamide (27)
White solid, m.p. 95–96 °C, Rf; 0.95 in ethylacetate/n-hexane (3/1), Yield; 98%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.83 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 8.09 (dd, J = 1.6 Hz, J = 4.5 Hz, 2 H, Ar-H), 7.83 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.65 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.41 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 3.49 (bs, 2 H, -CH2-), 3.20 (bs, 2 H, -CH2-), 1.72 (bs, 2 H, -CH2-), 1.55 (bs, 2 H, -CH2-), 1.00 (bs, 3 H, -CH3), 0.75 (bs, 2 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.6, 161.8, 150.8, 150.5, 142.2, 135.6, 134.0, 123.8, 121.0, 120.6, 109.6, 50.9, 21.9, 20.7, 11.4, 11.1. HRMS [M + H] (C19H22N3O2): Calculated: 324.1712; Found: 324.1728.
(2-(Pyridin-4-yl)benzo[doxazol-6-yl)(pyrrolidin-1-yl)methanone (28)
White solid, m.p. 144–145 °C, Rf; 0.60 in ethylacetate/n-hexane (1/1), Yield; 90%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.90–8.76 (m, 2 H, Ar-H), 8.08 (dd, J = 1.5 Hz, J = 4.6 Hz, 2 H, Ar-H), 7.83–7.79 (m, 2 H, Ar-H), 7.58 (dd, J = 1.4 Hz, J = 8.3 Hz, 1H, Ar-H), 3.68 (t, J = 6.8 Hz, 2 H, -CH2-), 3.47 (t, J = 6.8 Hz, 2 H, -CH2-), 2.02–1.96 (m, 2 H, -CH2-), 1.93–1.86 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 168.6, 162.0, 150.8, 150.4, 142.8, 135.4, 133.9, 124.5, 121.1, 120.4, 120.3, 110.3, 49.8, 46.4, 26.5, 24.5. HRMS [M + H] (C17H16N3O2): Calculated: 294.1243; Found: 294.1255.
Piperidin-1-yl(2-(pyridin-4-yl)benzo[d]oxazol-6-yl)methanone (29)
Gray solid, m.p. 127–128 °C, Rf; 0.75 in ethylacetate/n-hexane (2/1), Yield; 87%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.83 (d, J = 5.1 Hz, 2 H, Ar-H), 8.08 (dd, J = 5.1 Hz, J = 7.3 Hz, 2 H, Ar-H), 7.82 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.69 (dd, J = 0.6 Hz, J = 1.4 Hz, 1H, Ar-H), 7.44 (dd, J = 1.4 Hz, J = 8.2 Hz, 1H, Ar-H), 3.73 (bs, 2 H, -CH2-), 3.38 (bs, 2 H, -CH2-), 1.70–146 (m, 6 H). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.2, 161.9, 150.8, 150.5, 142.5, 134.7, 133.9, 124.1, 121.0, 120.6, 110.0, 49.0, 43.5, 26.4, 25.6, 24.5. HRMS [M + H] (C18H18N3O2): Calculated: 308.1399; Found: 308.1407.
Morpholino(2-(pyridin-4-yl)benzo[d]oxazol-6-yl)methanone (30)
Pink solid, m.p. 214–215 °C, Rf; 0.70 in ethylacetate/n-hexane (2/1), Yield; 73%. 1H-NMR (400 MHz, CDCl3) ppm δ = 8.85 (dd, J = 1.7 Hz, J = 4.5 Hz, 2 H, Ar-H), 8.09 (dd, J = 1.7 Hz, J = 4.5 Hz, 2 H, Ar-H), 7.85 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.73 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.46 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 4.01–3.94 (m, 1H), 3.85–3.52 (m, 7 H). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.4, 162.2, 150.9, 143.0, 133.8, 133.4, 124.3, 121.1, 120.8, 112.7, 110.5, 66.8, 52.5. HRMS [M + H] (C17H16N3O3): Calculated: 310.1192; Found: 310.1181.
N,N-dimethyl-2-(1 H-pyrrol-2-yl)benzo[d]oxazole-6-carboxamide (31)
Dark brown solid, m.p. 195–196 °C, Rf; 0.50 in ethylacetate/n-hexane (1/1), Yield; 52%. 1H-NMR (400 MHz, CDCl3) ppm δ = 10.62 (bs, 1H, -NH), 7.63 (dd, J = 3.0 Hz, J = 4.8 Hz, 2 H, Ar-H), 7.39 (dd, J = 1.4 Hz, J = 8.2 Hz, 1H, Ar-H), 7.13–7.09 (m, 1H, Ar-H), 7.06–7.02 (m, 1H, Ar-H), 6.40–6.35 (m, 1H, Ar-H), 3.14 (bs, 3 H, -CH3), 3.04 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.0, 159.4, 149.7, 143.0, 132.3, 124.0, 123.5, 119.4, 118.4, 113.9, 111.0, 109.8, 39.8, 35.6. HRMS [M + H] (C14H14N3O2): Calculated: 256.1086; Found: 256.1097.
N,N-diethyl-2-(1 H-pyrrol-2-yl)benzo[d]oxazole-6-carboxamide (32)
Brown solid, m.p. 137–138 °C, Rf; 0.70 in ethylacetate/n-hexane (3/1), Yield; 49%. 1H-NMR (400 MHz, CDCl3) ppm δ = 10.72 (bs, 1H, -NH), 7.62 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.57 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.34 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 7.13–7.08 (m, 1H, Ar-H), 7.06–7.01 (m, 1H, Ar-H), 6.37 (dd, J = 2.7 Hz, J = 3.6 Hz, 1H, Ar-H), 3.56 (bs, 2 H, -CH2-), 3.33 (bs, 2 H, -CH2-), 1.37–1.08 (m, 6 H, - CH3(2x)). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.7, 159.3, 149.7, 142.6, 133.3, 123.5, 123.2, 119.4, 118.6, 113.8, 111.0, 108.9, 43.4, 39.5, 14.1, 13.0. HRMS [M + H] (C16H18N3O2): Calculated: 284.1399; Found: 284.1409.
N,N-dipropyl-2-(1 H-pyrrol-2-yl)benzo[d]oxazole-6-carboxamide (33)
Brown viscous liquid, Rf; 0.90 in ethylacetate/n-hexane (3/1), Yield; 76%. 1H-NMR (400 MHz, CDCl3) ppm δ = 10.99 (bs, 1H, -NH), 7.61 (dd, J = 0.4 Hz, J = 8.1 Hz, 1H, Ar-H), 7.56 (d, J = 1.3 Hz, 1H, Ar-H), 7.31 (dd, J = 1.3 Hz, J = 8.1 Hz, 1H, Ar-H), 7.10 (dd, J = 1.3 Hz, J = 3.7 Hz, 1H, Ar-H), 7.05–6.99 (m, 1H, Ar-H), 7.06–7.01 (m, 1H, Ar-H), 6.36 (dd, J = 2.6 Hz, J = 3.7 Hz, 1H, Ar-H), 3.48 (bs, 2 H, -CH2-), 3.22 (bs, 2 H, -CH2-), 1.70 (bs, 2 H, -CH2-), 1.56 (bs, 2 H, -CH2-), 0.98 (bs, 3 H, -CH3), 0.74 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.2, 159.4, 149.7, 142.5, 133.4, 123.6, 123.4, 119.3, 118.5, 113.9, 110.9, 109.1, 50.9, 46.6, 21.9, 20.8, 11.3, 11.1. HRMS [M + H] (C18H22N3O2): Calculated: 312.1712; Found: 312.1725.
(2-(1 H-pyrrol-2-yl)benzo[d]oxazol-6-yl)(pyrrolidin-1-yl)methanone (34)
Light yellow solid, m.p. 207–208 °C, Rf; 0.80 in ethylacetate/n-hexane (1/1), Yield; 65%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.61 (bs, 1H, -NH), 7.72 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.64 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.52 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.13–7.09 (m, 1H, Ar-H), 7.08–7.05 (m, 1H, Ar-H), 6.41–6.37 (m, 1H, Ar-H), 3.69 (t, J = 7.0 Hz, 2 H, -CH2-), 3.50 (t, J = 6.5 Hz, 2 H, -CH2-), 2.03–1.95 (m, 2 H, -CH2-), 1.93–1.87 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.0, 159.1, 149.7, 143.2, 133.3, 124.1, 123.2, 119.5, 118.5, 113.6, 111.1, 109.7, 49.9, 46.4, 26.5, 24.5. HRMS [M + H] (C16H16N3O2): Calculated: 282.1243; Found: 282.1228.
(2-(1 H-pyrrol-2-yl)benzo[d]oxazol-6-yl)(piperidin-1-yl)methanone (35)
White solid, m.p. 215–216 °C, Rf; 0.82 in ethylacetate/n-hexane (1/1), Yield; 38%. 1H-NMR (400 MHz, CDCl3) ppm δ = 9.67 (bs, 1H, -NH), 7.64 (d, J = 8.1 Hz, 1H, Ar-H), 7.59 (d, J = 1.4 Hz, 1H, Ar-H), 7.37 (dd, J = 1.4 Hz, J = 8.1 Hz, 1H, Ar-H), 7.12–7.10 (m, 1H, Ar-H), 7.07–7.06 (m, 1H, Ar-H), 6.40–6.38 (m, 1H, Ar-H), 3.80–3.64 (m, 2 H, -CH2-), 3.47–3.33 (m, 2 H, -CH2-), 1.74–1.65 (m, 6 H, -CH2-(x3)). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 149.8, 142.9, 132.6, 123.7, 123.2, 119.5, 118.7, 118.3, 113.6, 111.1, 109.5, 47.9, 43.5, 31.6, 26.9, 24.6. HRMS [M + H] (C17H18N3O2): Calculated: 296.1399; Found: 296.1412.
(2-(1 H-pyrrol-2-yl)benzo[d]oxazol-6-yl)(morpholino)methanone (36)
Orange solid, m.p. 135–136 °C, Rf; 0.65 in ethylacetate/n-hexane (1/1), Yield; 44%. 1H-NMR (400 MHz, CDCl3) ppm δ = 12.33 (bs, 1H, -NH), 7.74 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.69 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.38 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 7.15–7.11 (m, 1H, Ar-H), 6.99 (dd, J = 1.4 Hz, J = 3.6 Hz, 1H, Ar-H), 6.29 (dd, J = 2.5 Hz, J = 3.6 Hz, 1H, Ar-H), 3.72–3.44 (m, 8 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.0, 159.4, 149.5, 143.3, 131.9, 125.1, 124.5, 118.9, 118.8, 113.9, 110.8, 110.0, 66.5. HRMS [M + H] (C16H16N3O3): Calculated: 298.1192; Found: 298.1224.
2-(Furan-2-yl)-N,N-dimethylbenzo[d]oxazole-6-carboxamide (37)
Light brown solid, m.p. 94–95 °C, Rf; 0.84 in ethylacetate/n-hexane (1/1), Yield; 86%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.72 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.66 (dd, J = 0.8 Hz, J = 1.8 Hz, 1H, Ar-H), 7.63 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.40 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.28 (dd, J = 0.8 Hz, J = 3.5 Hz, 1H, Ar-H), 6.60 (dd, J = 1.8 Hz, J = 3.5 Hz, 1H, Ar-H), 3.11 (bs, 3 H, -CH3), 3.00 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.7, 156.5, 149.7, 146.1, 142.7, 142.2, 133.4, 124.2, 119.8, 115.0, 112.4, 109.9, 39.7, 35.5. HRMS [M + H] (C14H13N2O3): Calculated: 257.0926; Found: 257.0944.
N,N-diethyl-2-(furan-2-yl)benzo[d]oxazole-6-carboxamide (38)
Dark brown solid, m.p. 101–102 °C, Rf; 0.75 in ethylacetate/n-hexane (1/2), Yield; 79%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.73 (dd, J = 0.7 Hz, J = 8.1 Hz, 1H, Ar-H), 7.67–7.65 (m, 1H, Ar-H), 7.59–7.57 (m, 1H, Ar-H), 7.36 (dd, J = 1.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.29–7.27 (m, 1H, Ar-H), 6.60 (dd, J = 1.6 Hz, J = 3.5 Hz, 1H, Ar-H), 3.54 (bs, 2 H, -CH2-), 3.28 (bs, 2 H, -CH2-), 1.22 (bs, 3 H, -CH3), 1.07 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.4, 156.3, 149.8, 146.1, 142.3, 142.2, 134.4, 123.4, 120.0, 114.9, 112.4, 109.1, 43.4, 39.5, 14.1, 12.9. HRMS [M + H] (C16H17N2O3): Calculated: 285.1239; Found: 285.1251.
2-(Furan-2-yl)-N,N-dipropylbenzo[d]oxazole-6-carboxamide (39)
Light Brown viscous liquid, Rf; 0.88 in ethylacetate/n-hexane (1/3), Yield; 94%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.71 (dd, J = 0.58 Hz, J = 8.13 Hz, 1H, Ar-H), 7.65 (dd, J = 0.78 Hz, J = 1.75 Hz, 1H, Ar-H), 7.55 (dd, J = 0.58 Hz, J = 1.45 Hz, 1H, Ar-H), 7.33 (dd, J = 1.45 Hz, J = 8.13 Hz, 1H, Ar-H), 7.27 (dd, J = 0.78 Hz, J = 3.54 Hz, 1H, Ar-H), 6.59 (dd, J = 1.75 Hz, J = 3.54 Hz, 1H, Ar-H), 3.44 (bs, 2 H, -CH2-), 3.16 (bs, 2 H, -CH2-), 1.67 (bs, 2 H, -CH2-), 1.52 (bs, 2 H, -CH2-), 0.95 (bs, 3 H, -CH3), 0.70 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.8, 156.3, 149.8, 149.7, 146.1, 142.2, 134.5, 123.6, 120.0, 114.9, 112.4, 109.3, 50.8, 46.5, 21.9, 20.7, 11.4, 11.0. HRMS [M + H] (C18H21N2O3): Calculated: 313.1552; Found: 313.1518.
(2-(Furan-2-yl)benzo[doxazol-6-yl)(pyrrolidin-1-yl)methanone (40)
White solid, m.p. 98–100 °C, Rf; 0.90 in ethylacetate/n-hexane (1/1), Yield; 65%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.77–7.74 (m, 2 H, Ar-H), 7.70 (dd, J = 0.8 Hz, J = 1.8 Hz, 1H, Ar-H), 7.55 (dd, J = 1.4 Hz, J = 8.3 Hz, 1H, Ar-H), 7.32 (dd, J = 0.8 Hz, J = 3.5 Hz, 1H, Ar-H), 6.64 (dd, J = 1.8 Hz, J = 3.5 Hz, 1H, Ar-H), 3.68 (t, J = 6.8 Hz, 2 H, -CH2-), 3.48 (t, J = 6.8 Hz, 2 H, -CH2-), 2.01–1.96 (m, 2 H, -CH2-), 1.93–1.87 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 168.8, 156.6, 149.7, 146.1, 142.9, 142.3, 134.4, 124.3, 119.7, 115.0, 112.4, 110.0, 49.9, 46.4, 26.5, 24.4. HRMS [M + H] (C16H15N2O3): Calculated: 283.1083; Found: 283.1107.
(2-(Furan-2-yl)benzo[doxazol-6-yl)(piperidin-1-yl)methanone (41)
Light Brown viscous liquid, Rf; 0.90 in ethylacetate/n-hexane (1/1), Yield; 87%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.76 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.69 (dd, J = 0.8 Hz, J = 1.8 Hz, 1H, Ar-H), 7.63 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.40 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.32 (dd, J = 0.8 Hz, J = 3.5 Hz, 1H, Ar-H), 6.64 (dd, J = 1.8 Hz, J = 3.5 Hz, 1H, Ar-H), 3.81–3.65 (bs, 2 H, -CH2-), 3.46–3.33 (bs, 2 H, -CH2-), 1.75–1.65 (m, 2 H, -CH2-), 1.61–1.51 (bs, 4 H, -CH2-(x2)). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.5, 156.4, 149.8, 146.1, 142.6, 142.3, 133.6, 123.9, 120.0, 115.0, 112.4, 109.7, 49.1, 43.5, 29.7, 26.5, 24.6. HRMS [M + H] (C17H17N2O3): Calculated: 297.1239; Found: 297.1244.
(2-(Furan-2-yl)benzo[doxazol-6-yl)(morpholino)methanone (42)
Light orange solid, m.p. 108–109 °C, Rf; 0.72 in ethylacetate/n-hexane (1/1), Yield; 81%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.78 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.70 (dd, J = 0.8 Hz, J = 1.8 Hz, 1H, Ar-H), 7.66 (0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.42 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 7.33 (dd, J = 0.8 Hz, J = 3.5 Hz, 1H, Ar-H), 6.65 (dd, 1.8 Hz, J = 3.5 Hz, 1H, Ar-H), 3.88–3.36 (m, 8 H, -CH2-(x4)). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 156.7, 149.9, 146.2, 143.0, 142.1, 132.3, 124.2, 120.1, 115.2, 112.5, 110.1, 66.9. HRMS [M + H] (C16H15N2O4): Calculated: 299.1032; Found: 299.1061.
N,Ndimethyl-2-(thiophen-2-yl)benzo[d]oxazole-6-carboxamide (43)
Light yellow solid, m.p.128–129 °C, Rf; 0.84 in ethylacetate/n-hexane (1/1), Yield; 78%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.93 (dd, J = 1.2 Hz, J = 3.8 Hz, 1H, Ar-H), 7.72 (d, J = 8.2 Hz, 1H, Ar-H), 7.64 (d, J = 1.2 Hz, 1H, Ar-H), 7.58 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, Ar-H), 7.41 (dd, J = 1.2 Hz, J = 8.2 Hz, 1H, Ar-H), 7.19 (dd, J = 3.8 Hz, J = 5.0 Hz, 1H, Ar-H), 3.14 (bs, 3 H, -CH3), 3.02 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.8, 160.3, 150.0, 143.1, 133.2, 130.9, 130.5, 129.2, 128.4, 124.1, 119.5, 109.8, 39.8, 35.6. HRMS [M + H] (C14H13N2O2S): Calculated: 273.0698; Found: 273.0729.
N,N-2-(thiophen-2-yl)benzo[d]oxazole-6-carboxamide (44)
Brown solid, m.p. 130–131 °C, Rf; 0.95 in ethylacetate/n-hexane (1/1), Yield; 71%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.93 (dd, J = 1.4 Hz, J = 3.7 Hz, 1H, Ar-H), 7.73 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.60–7.58 (m, 2 H, Ar-H), 7.37 (dd, J = 1.4 Hz, J = 8.1 Hz, 1H, Ar-H), 7.20 (dd, J = 3.7 Hz, J = 5.0 Hz, 1H, Ar-H), 3.56 (bs, 2 H, -CH2-), 3.32 (bs, 2 H, -CH2-), 1.29–1.11 (m, 6 H, -CH3(2x)). 13C-NMR (100 MHz, CDCl3) ppm δ = 170.5, 160.2, 150.1, 142.7, 134.2, 130.8, 130.4, 129.2, 128.3, 123.3, 119.7, 109.0, 43.4, 39.6, 14.1, 13.1. HRMS [M + H] (C16H17N2O2S): Calculated: 301.1011; Found: 301.1040.
N,Ndipropyl-2-(thiophen-2-yl)benzo[d]oxazole-6-carboxamide (45)
Dark yellow viscous liquid, Rf; 0.96 in ethylacetate/n-hexane (3/1), Yield; 85%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.92 (dd, J = 1.22 Hz, J = 3.75 Hz, 1H, Ar-H), 7.71 (d, J = 8.15 Hz, 1H, Ar-H), 7.58–7.56 (m, 2 H, Ar-H), 7.34 (dd, J = 1.45 Hz, J = 8.15 Hz, 1H, Ar-H), 7.19 (dd, J = 3.75 Hz, J = 4.95 Hz, 1H, Ar-H), 3.47 (bs, 2 H, -CH2-), 3.19 (bs, 2 H, -CH2-), 1.70 (bs, 2 H, -CH2-), 1.54 (bs, 2 H, -CH2-), 0.99 (bs, 3 H, -CH3), 0.73 (bs, 3 H, -CH3). 13C-NMR (100 MHz, CDCl3) ppm δ = 171.0, 160.1, 150.1, 142.6, 134.3, 130.8, 130.4, 129.2, 128.4, 123.5, 119.6, 109.2, 50.9, 46.5, 21.9, 20.7, 11.4, 11.1. HRMS [M + H] (C18H21N2O2S): Calculated: 329.1324; Found: 329.1310.
Pyrrolidin-1-yl(2-(thiophen-2-yl)benzo[d]oxazol-6-yl)methanone (46)
Light brown solid, m.p. 140–141 °C, Rf; 0.80 in ethylacetate/n-hexane (1/1), Yield; 80%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.94 (dd, J = 1.2 Hz, J = 3.8 Hz, 1H, Ar-H), 7.76–7.68 (m, 2 H, Ar-H), 7.60 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, Ar-H), 7.53 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.21 (dd, J = 3.8 Hz, J = 5.0 Hz, 1H, Ar-H), 3.69 (t, J = 6.8 Hz, 2 H, -CH2-), 3.48 (t, J = 6.8 Hz, 2 H, -CH2-), 2.04–1.86 (m, 4 H, -CH2-(x2)). 13C-NMR (100 MHz, CDCl3) ppm δ = 168.9, 160.4, 150.0, 143.2, 134.1, 130.9, 130.5, 129.2, 128.4, 124.2, 119.4, 109.9, 49.9, 46.4, 26.5, 24.5. HRMS [M + H] (C16H15N2O2S): Calculated: 299.0854; Found: 299.0871.
Piperidin-1-yl(2-(thiophen-2-yl)benzo[d]oxazol-6-yl)methanone (47)
Light yellow solid, m.p.151–152 °C, Rf; 0.60 in ethylacetate/n-hexane (1/2), Yield; 75%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.94 (dd, J = 1.2 Hz, J = 3.7 Hz, 1H, Ar-H), 7.73 (dd, J = 0.6 Hz, J = 8.2 Hz, 1H, Ar-H), 7.62 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.59 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, Ar-H), 7.39 (dd, J = 1.5 Hz, J = 8.2 Hz, 1H, Ar-H), 7.21 (dd, J = 3.7 Hz, J = 5.0 Hz, 1H, Ar-H), 3.72 (bs, 2 H, -CH2-), 3.40 (bs, 2 H, -CH2-), 1.75–1.66 (m, 4 H,-CH2-(x2)), 1.64–1.55 (m, 2 H, -CH2-). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.6, 160.3, 150.1, 143.0, 133.4, 130.8, 130.5, 129.2, 128.4, 123.8, 119.6, 109.6, 48.9, 43.4, 26.1, 25.5, 24.6. HRMS [M + H] (C17H17N2O2S): Calculated: 313.1012; Found: 313.1059.
Morpholino(2-(thiophen-2-yl)benzo[d]oxazol-6-yl)methanone (48)
Brown solid, m.p. 213–214 °C, Rf; 0.85 in ethylacetate/n-hexane (2/1), Yield; 82%. 1H-NMR (400 MHz, CDCl3) ppm δ = 7.95 (dd, J = 1.2 Hz, J = 3.8 Hz, 1H, Ar-H), 7.75 (dd, J = 0.6 Hz, J = 8.1 Hz, 1H, Ar-H), 7.65 (dd, J = 0.6 Hz, J = 1.5 Hz, 1H, Ar-H), 7.61 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, Ar-H), 7.41 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H, Ar-H), 7.21 (dd, J = 3.8 Hz, J = 5.0 Hz, 1H, Ar-H), 3.84–3.55 (m, 8 H, -CH2-(x4)). 13C-NMR (100 MHz, CDCl3) ppm δ = 169.7, 160.5, 150.1, 143.4, 132.0, 131.1, 130.7, 129.0, 128.4, 124.1, 119.8, 110.0, 66.9. HRMS [M + H] (C16H15N2O3S): Calculated: 315.0803; Found: 315.0788.
Biochemistry
In the present work, the in vitro effects of 2-aryl-6-carboxamide benzoxazole derivatives (7–48) on AChE/BChE from electric eel (Electrophorus electricus)/equine serum was realized according to Ellman’s method (1961) as previously described [54]. These analogs were dissolved in DMSO at an initial concentration of 1 mg/mL. Around 1% of DMSO was present in the final reaction mixture. Acetylthiocholine iodide (AChI)/butyrylcholine iodide (BChI) were used as substrates for enzymatic reactions. For this aim, an aliquot (0.1 mL) of Tris/HCl buffer (pH 8.0, 1.0 M) and different 2-aryl-6-carboxamide benzoxazole derivatives (7–48) concentrations (10–30 µg/mL) were added to 50 µL of AChE/BChE solution (5.30 × 10− 3 EU). The mixture was incubated at 20 oC for 20 min. Then, 50 µL of 5,5’-dithio-bis 2-nitro-benzoic acid (DTNB, 0.5 mM) and AChI/BChI were added and enzymatic reactions were initiated. Then, AChE/BChE activities were spectrophotometrically determined at 412 nm [55].
AChE and BChE inhibition assay
The half maximal inhibition concentration (IC50) was calculated from activity (%) versus 2-aryl-6-carboxamide benzoxazole derivatives (7–48) plots [56]. Lineweaver-Burk graphs were used for the calculation of Ki and the other parameters as described in prior studies [57]. The Ki values were taken out from this graph [58]. All analyses were conducted independently in triplicate, and result data are expressed as mean values ± SD [59].
Molecular docking
Molecular docking studies were carried out with Maestro (Schrodinger, 12.8.117). Compound 36, which is the most active compound against AChE and BChE, was chosen for docking studies. The crystal structure of 1EVE pdb-encoded protein of the complex of donepezil in AChE was downloaded from https://www.rcsb.org/. The homology model of Equus caballus (horse) BChE was built [50, 60]. There is no crystal structure available for ECBChE. For this reason, a homology model of Equus caballus (horse) BChE (ECBChE) was constructed utilizing comparative modeling techniques. In homology modeling, Q9N1N9 was used as the sequence for ECBChE, and 4TPK was used as a template structure. Homology modeling was performed by simply aligning the sequences of these enzymes with the most homologous sequences of the proteins with available 3D structure, hBChE (homo sapiens), and threading the target model structures. Sequence identity between ECBChE and hBChE, co-crystallized ligand (3F9) in the active site of hBChE, and solvent molecules were transferred to the respective model structures using a specific MODELLER script. The structural quality of the model was confirmed by the PROCHECK analyses [50].
Water molecules, heteroatoms, and ligands in the structure of proteins have been removed, and hydrogen atoms were added to the protein by using Protein Preparation Wizard module. In this method, the ionization and tautomeric states were created by Epik (Schrodinger, 12.8.117), whereas the proton orientations were determined by PROPKA. Impact (Schrodinger, 12.8.117) was used to perform constrained reduction with a convergence of heavy atoms to an RMSD of 3 Å. The structures have become suitable for protein docking studies. The 20 Å sphere was identified as binding sites at active sites of proteins using the Receptor Grid Generation module. The 3D structures of the ligands were created with MacroModel. Ligands were prepared using LigPrep to include possible ionization and tautomeric states and optimized using OPLS4 force field and conjugate gradient method. Ligands were docked to the binding sites with standard precision (sp) 100 using the Glide program with default settings, and the docking scores were calculated [48, 49]. To confirm the validity of the docking procedure, E20 (donepezil) and 3F9 were re-docked to their respective binding sites; the RMSD values of the resulting docking postures were 0.80 and 0.91, respectively. Maestro (Schrodinger, 12.8.117) and the workspace ligand interaction diagram module were used to create 2D and 3D figures.
Molecular dynamics simulations
Desmond (Schrodinger, 12.8.117) program was used in all molecular dynamics simulations. Molecular dynamics simulations were performed for 100 ns under NPT ensemble, for compound 36, the most active compound against AChE and BChE. In these simulations, average RMSD values of ligands and proteins and interactions of ligands with residues were investigated. Ligands were solvated in an octahedral box with TIP3P water molecules leaving at least 10 Å. The systems were neutralized by Na+ and Cl− counter ions and the concentrations of the systems were adjusted with 0.15 M NaCl solution. Desmond’s standard relaxation methodology was used, and before production simulations, prepared solvated simulation systems were first put through a series of minimizations and short MD simulations [49, 60]. The Nose-Hoover thermostat was used to regulate the systems’ temperature during MD simulations, and it was initially set to 300 K. With isotropic pressure coupling, the Martyna-Tobias-Klein method was used to regulate pressure around 1.01325 bar. 9 Å cutoff for the short-range nonbonded interactions was used combined with the particle mesh Ewald option [59,60,61,62].
ADME study
Some predicted pharmacokinetic properties of synthesised compounds used in absorption, distribution, metabolism, and excretion (ADME) analyzes were calculated using QikProp (Maestro, Schrodinger, 12.8.117) and SwissADME http://www.swissadme.ch/ software [63].
References
Sharma P, Srivastava P, Seth A, Tripathi PN, Banerjee AG, Shrivastava SK (2019) Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2018.12.006
Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA (2004) Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2634794100
Patterson C, World Alzheimer Report https://apo.org.au/node/260056 (accessed 12 October 2022). 4., Giacobini E (2018) (2003) Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem Res. https://doi.org/10.1023/A:1022869222652
Giacobini E (2003) Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem Res 28(3/4):515–522. https://doi.org/10.1023/A:1022869222652
Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. https://doi.org/10.1126/science.1678899
Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M (2014) Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. https://doi.org/10.1111/joim.12191
Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F (2012) New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol. https://doi.org/10.1111/j.1365-2125.2011.04134.x
Eagger SA, Levy R, Sahakian BJ (1991) Tacrine in Alzheimer’s disease. Lancet. https://doi.org/10.1016/0140-6736(91)92656-M
Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N (2017) Memantine for Alzheimer’s Disease: an updated systematic review and Meta-analysis. J Alzheimer’s Dis. https://doi.org/10.3233/JAD-170424
Birks JS, Evans JG (2015) Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001191.pub3
Prvulovic D, Pantel J (2010) Galantamine for Alzheimer’s disease. Expert Opin Drug Metab Toxicol. https://doi.org/10.1517/17425251003592137
Benjamin B, Burns A (2007) Donepezil for Alzheimer’s disease. Expert Rev Neurother. https://doi.org/10.1586/14737175.7.10.1243
Qizilbash N, Birks J, Lopez AJ, Lewington S, Szeto S (2005) Tacrine for Alzheimer’s disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000202
Kazmierski J, Messini-Zachou C, Gkioka M, Tsolaki M (2018) The impact of a long-term rivastigmine and donepezil treatment on all-cause mortality in patients with Alzheimer’s disease. Am J Alzheimer’s Dis Other Dement. https://doi.org/10.1177/1533317518775044
Oset-Gasque MJ, Marco-Contelles J (2018) Alzheimer’s Disease, the One-Molecule, one-target paradigm, and the Multitarget Directed ligand Approach. ACS Chem Neurosci. https://doi.org/10.1021/acschemneuro.8b00069
Mishra P, Kumar A, Panda G (2019) Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). https://doi.org/10.1016/j.bmc.2019.01.025. Bioorg Med Chem
Zha X, Lamba D, Zhang L, Lou Y, Xu C, Kang D, Chen L, Xu Y, Zhang L, De Simone A, Samez S, Pesaresi A, Stojan J, Lopez MG, Egea J, Andrisano V, Bartolini M (2016) Novel tacrine–Benzofuran hybrids as Potent Multitarget-Directed ligands for the treatment of Alzheimer’s Disease: design, synthesis, Biological evaluation, and X-ray crystallography. J Med Chem. https://doi.org/10.1021/acs.jmedchem.5b01119
Elsinghorst PW, Cieslik J, Mohr K, Tränkle C, Gütschow M (2007) First gallamine – tacrine hybrid: design and characterization at Cholinesterases and the M2 muscarinic receptor. J Med Chem. https://doi.org/10.1021/jm070859s
Shao D, Zou C, Luo C, Tang X, Li Y (2004) Synthesis and evaluation of tacrine–E2020 hybrids as acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2004.07.005
Alonso D, Dorronsoro I, Rubio L, Muñoz P, García-Palomero E, Del Monte M, Bidon-Chanal A, Orozco M, Luque FJ, Castro A, Medina M, Martínez A (2005) Donepezil–tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg Med Chem. https://doi.org/10.1016/j.bmc.2005.09.029
Camps P, Formosa X, Galdeano C, Gómez T, Muñoz-Torrero D, Scarpellini M, Viayna E, Badia A, Clos MV, Camins A, Pallàs M, Bartolini M, Mancini F, Andrisano V, Estelrich J, Lizondo M, Bidon-Chanal A, Luque FJ (2008) Novel donepezil-based inhibitors of Acetyl- and Butyrylcholinesterase and Acetylcholinesterase-Induced β-Amyloid aggregation. J Med Chem. https://doi.org/10.1021/jm8001313
Keri RS, Quintanova C, Marques SM, Esteves AR, Cardoso SM, Santos MA (2013) Design, synthesis and neuroprotective evaluation of novel tacrine–benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg Med Chem. https://doi.org/10.1016/j.bmc.2013.05.028
Telpoukhovskaia MA, Patrick BO, Rodríguez-Rodríguez C, Orvig C (2013) Exploring the multifunctionality of thioflavin- and deferiprone-based molecules as acetylcholinesterase inhibitors for potential application in Alzheimer’s disease. Mol Biosyst. https://doi.org/10.1039/C3MB25600F
Agatonovic-Kustrin S, Kettle C, Morton DW (2018) A molecular approach in drug development for Alzheimer’s disease. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.06.147
Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C (2008) Multi-target-directed Ligands to combat neurodegenerative diseases. J Med Chem. https://doi.org/10.1021/jm7009364
Bottegoni G, Favia AD, Recanatini M, Cavalli A (2012) The role of fragment-based and computational methods in polypharmacology. Drug Discov. https://doi.org/10.1016/j.drudis.2011.08.002
Kramer T, Schmidt B, Lo Monte F (2012) Small-molecule inhibitors of GSK-3: structural insights and their application to Alzheimer’s Disease models. J Alzheimer’s Dis. https://doi.org/10.1155/2012/381029
Branduardi D, Gervasio FL, Cavalli A, Recanatini M, Parrinello M (2005) The role of the peripheral anionic site and cation – π interactions in the ligand penetration of the human AChE gorge. J Am Chem Soc. https://doi.org/10.1021/ja0512780
Youssef KM, Fawzy IM, El-Subbagh HI (2018) N-substituted-piperidines as Novel Anti-alzheimer agents: synthesis, antioxidant activity, and molecular docking study. Future J Pharm Sci. https://doi.org/10.1016/j.fjps.2017.06.002
Silva D, Chioua M, Samadi A, Agostinho P, Garção P, Lajarín-Cuesta R, de los Ríos C, Iriepa I, Moraleda I, Gonzalez-Lafuente L, Mendes E, Pérez C, Rodríguez-Franco MI, Marco-Contelles J, Carreiras MC (2013) Synthesis, pharmacological Assessment, and Molecular modeling of Acetylcholinesterase/Butyrylcholinesterase inhibitors: effect against Amyloid-β-Induced neurotoxicity. ACS Chem Neurosci. https://doi.org/10.1021/cn300178k
Simoes MCR, Viegas FPD, Moreira MS, Silva MF, Riquiel MM, Rosa PM, Castelli MR, Santos MH, Soares MG, Viegas C (2014) Donepezil: an important prototype to the design of new drug candidates for Alzheimer’s disease. Mini-Rev Med Chem. https://doi.org/10.2174/1389557513666131119201353
Lan JS, Zhang T, Liu Y, Yang J, Xie SS, Liu J, Miao ZY, Ding Y (2017) Design, synthesis and biological activity of novel donepezil derivatives bearing N-benzyl pyridinium moiety as potent and dual binding site acetylcholinesterase inhibitors. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2017.02.045
Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ (2012) Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. https://doi.org/10.1021/jm300871x
Huang CS, Tu WT, Luo M, Shi JCMD (2016) Molecular Docking and Design of Novel heterodimers of Donepezil and Huperzine fragments as acetylcholinesterase inhibitors. Chin J Chem. https://doi.org/10.14102/j.cnki.0254-5861.2011-0733
Pouramiri B, Mahdavi S, Nadri H, Moradi A, Tavakolinejad-Kermani E, Foroumadi A (2017) Synthesis and anticholinesterase activity of new substituted benzo[d]oxazole-based derivatives. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.12902
Celik I, Erol M, Temiz-Arpaci O, Senol FS, Orhan IE (2022) Evaluation of activity of some 2,5-Disubstituted benzoxazole derivatives against acetylcholinesterase, butyrylcholinesterase and tyrosinase: ADME Prediction, DFT and comparative molecular Docking studies. https://doi.org/10.1080/10406638.2020.1737827. Polycycl Aromat Compd
Temiz-Arpaci O, Arisoy M, Sac D, Doganc F, Tasci M, Senol FS (2016) Biological evaluation and docking studies of some benzoxazole derivatives as inhibitors of acetylcholinesterase and butyrylcholinesterase. Z fur Naturforsch -. https://doi.org/10.1515/znc-2016-0087. C J Biosci
Gulçin İ, Abbasova M, Taslimi P, Huyut Z, Safarova L, Sujayev A, Farzaliyev V, Beydemir S, Alwasel SH, Supuran CT (2017) Synthesis and biological evaluation of aminomethyl and alkoxymethyl derivatives as carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase inhibitors. J Enzyme Inhib Med Chem. https://doi.org/10.1080/14756366.2017.1368019
Akıncıoğlu H, Gülçin İ (2020) Potent acetylcholinesterase inhibitors: potential drugs for Alzheimer’s Disease. Mini-Rev Med Chem. https://doi.org/10.2174/1389557520666200103100521
Yigit M, Celepci DB, Taslimi P, Yiğit B, Çetinkaya E, Özdemir İ, Aygün M, Gülçin İ (2022) Selenourea and Thiourea derivatives of chiral and achiral enetetramines: synthesis, characterization and enzyme inhibitory properties. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105566
Takım K, Yigin A, Koyuncu İ, Kaya R, Gulçin İ (2021) Anticancer, anticholinesterase and antidiabetic activities of tunceli garlic (Allium tuncelianum): determining its phytochemical content by LC–MS/MS analysis. https://doi.org/10.1007/s11694-021-00912-y. J Food Meas Charact
Gunsel A, Yıldırım A, Taslimi P, Erden Y, Taskin-Tok T, Pişkin H, Bilgiçli AT, Gülçin İ, Yarasir MN (2022) Cytotoxicity effects and biochemical investigation of novel tetrakis-phthalocyanines bearing 2-thiocytosine moieties with molecular docking studies. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2022.109263
Gülçin İ, Scozzafava A, Supuran CT, Akıncıoglu H, Koksal Z, Turkan F, Alwasel S (2016) The effect of caffeic acid phenethyl ester (CAPE) on metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione S-transferase, lactoperoxidase, and carbonic anhydrase isoenzymes I, II, IX, and XII. J Enzyme Inhib Med Chem. https://doi.org/10.3109/14756366.2015.1094470
Yuksel M, Biberoglu K, Onder S, Akbulut KG, Tacal O (2018) Toluidine blue O modifies hippocampal amyloid pathology in a transgenic mouse model of Alzheimer’s disease. https://doi.org/10.1016/j.biochi.2017.12.004. Biochimie
Vrolix K, Fraussen J, Losen M, Stevens J, Lazaridis K, Molenaar PC, Somers V, Bracho MA, Le Panse R, Stinissen P, Berrih-Aknin S, Maessen JG, Van Garsse L, Buurman WA, Tzartos SJ, De Baets MH, Martinez-Martinez P (2014) Clonal heterogeneity of thymic B cells from early-onset myasthenia gravis patients with antibodies against the acetylcholine receptor. J Autoimmun. https://doi.org/10.1016/j.jaut.2013.12.008
Perry EK, Perry RH, Blessed G, Tomlinson BE (1978) Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. https://doi.org/10.1111/j.1365-2990.1978.tb00545.x
Dighe SN, Deora GS, De la Mora E, Nachon F, Chan S, Parat MO, Ross BP (2016) Discovery and structure–activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J Med Chem. https://doi.org/10.1021/acs.jmedchem.6b00356
Özçelik AB, Özdemir Z, Sari S, Utku S, Uysal M (2019) A new series of pyridazinone derivatives as cholinesterases inhibitors: synthesis, in vitro activity and molecular modeling studies. Pharmacol Rep. https://doi.org/10.1016/j.pharep.2019.07.006
Anil DA, Polat MF, Saglamtas R, Tarikogullari AH, Alagoz MA, Gulcin I, Algul O (2022) Exploring enzyme inhibition profiles of novel halogenated chalcone derivatives on some metabolic enzymes: synthesis, characterization and molecular modeling studies. Comput Biol Chem. https://doi.org/10.1016/j.compbiolchem.2022.107748
Galdeano C, Arroyo P, Bidon-Chanal A, Blas JR, Munoz-Torrero D, Luque FJ (2010) Structural determinants of the Multifunctional Profile of dual binding site acetylcholinesterase inhibitors as Anti-alzheimer agents. https://doi.org/10.2174/138161210793176536. Curr Pharm Des
Kuzu B, Sari O, Erdem SS, Algul O, Menges N (2021) Synthesis of Benzoxazole-2-carboxylate Derivatives: electronic- and position-effect of functional groups and computational modeling of the selectivity for Oxazole Ring. https://doi.org/10.1002/slct.202100174. ChemistrySelect
Kuzu B, Hepokur C, Turkmenoglu B, Burmaoglu S, Algul O (2022) Design, synthesis and in vitro antiproliferation activity of some 2-aryl and -heteroaryl benzoxazole derivatives. Fut Med Chem. https://doi.org/10.4155/fmc-2022-0076
Kuzu B, Ayaz F, Algul O (2019) Synthesis of New Alicyclic Oxalamide derivatives and their Differential Immunomodulatory activities on the mammalian cells. J Heterocycl Chem. https://doi.org/10.1002/jhet.3573
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. https://doi.org/10.1016/0006-2952(61)90145-9
Erdemir F, Celepci DB, Aktaş A, Gök Y, Kaya R, Taslimi P, Demir Y, Gulçin İ (2019) Novel 2-aminopyridine liganded pd(II) N-heterocyclic carbene complexes: synthesis, characterization, crystal structure and bioactivity properties. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2019.103134
Caglayan C, Taslimi P, Türk C, Gulcin İ, Kandemir FM, Demir Y, Beydemir Ş (2020) Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-07611-z
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc. https://doi.org/10.1021/ja01318a036
Dasgin S, Gok Y, Celepci DB, Taslimi P, İzmirli M, Aktaş A, Gülçin İ (2021) Synthesis, characterization, crystal structure and bioactivity properties of the benzimidazole-functionalized PEPPSI type of pd(II)NHC complexes. https://doi.org/10.1016/j.molstruc.2020.129442. J Mol Struct
Gulçin I, Alwasel SH (2022) Metal ions, metal chelators and metal chelating assay as antioxidant method. https://doi.org/10.3390/pr10010132. Processes
Ozten O, Kurt BZ, Sonmez F, Dogan B, Durdagi S (2021) Synthesis, molecular docking and molecular dynamics studies of novel tacrine-carbamate derivatives as potent cholinesterase inhibitors. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105225
Nose S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. https://doi.org/10.1103/PhysRevA.31.1695
Martyna GJ, Tobias DJ, Klein ML (1994) Constant pressure molecular dynamics algorithms. J Chem Phys. https://doi.org/10.1063/1.467468
Askin S, Tahtaci H, Türkeş C, Demir Y, Ece A, Akalın Çiftçi G, Beydemir Ş (2021) Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo[2,1-b][1,3,4]thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105009
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Burak Kuzu, M. Abdullah Alagoz, Yeliz Demir: Methodology, Data curation,Burak Kuzu, M. Abdullah Alagoz, Ilhami Gulcin, Serdar Burmaoglu: Writing – original draft. Burak Kuzu, M. Abdullah Alagoz, Serdar Burmaoglu: Investigation, Programming, Supervision. Burak Kuzu, M. Abdullah Alagoz, Yeliz Demir, Ilhami Gulcin, Serdar Burmaoglu: Visualization, Validation. All authors: Writing – review for the final version of the manuscriptOztekin Algul: Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuzu, B., Alagoz, M.A., Demir, Y. et al. Structure-based inhibition of acetylcholinesterase and butyrylcholinesterase with 2-Aryl-6-carboxamide benzoxazole derivatives: synthesis, enzymatic assay, and in silico studies. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10828-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10828-6