Abstract
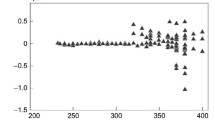
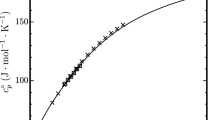
This study discusses the transition toward the use of environmentally friendly refrigerants in low-temperature technology. It also considers a new environmentally friendly fourth-generation refrigerant trans‑1,3,3,3-tetrafluoropropene R1234ze(E) as an alternative to the R134a refrigerant common in chillers and heat pumps, as well as the R22 refrigerant in air conditioning systems. A technique has been developed for constructing a unified fundamental equation on the states of liquid and gas for trans‑1,3,3,3-tetrafluoropropene. Around the critical point, the proposed fundamental equation satisfies the requirements of the scale theory for asymmetric systems, and in the region of a rarefied gas, it is reduced to the virial equation of state. Based on this fundamental equation, tables of standard reference data on pressure, density, enthalpy, isobaric and isochoric heat capacities, entropy, heat of vaporization, and sound velocity of trans‑1,3,3,3-tetrafluoropropene in the region of state parameters in the temperature ranges of 169–420 K and pressure of 0.1–100 MPa were calculated. Several statistical characteristics have been calculated, namely, absolute mean deviation, systematic deviation, standard deviation, and root mean square deviation, characterizing the accuracy of the proposed fundamental equation in describing the experimental values of the equilibrium properties obtained in recognized international thermophysical centers. Evidently, the values of these statistical characteristics are significantly lesser than those of the corresponding characteristics of the international fundamental equations of state, provided in the literature, when describing the thermal and caloric experimental data of trans‑1,3,3,3-tetrafluoropropene. The estimated expanded uncertainties of the tabulated data based on the proposed fundamental equation were 0.26% for density, 0.57% for pressure, 1.7% and 1.2% for isochoric and isobaric heat capacities, respectively, and 0.38% for sound velocity. The results suggest that the proposed unified fundamental equation of state adequately conveys the thermodynamic characteristics of trans‑1,3,3,3-tetrafluoropropene over the specified range of temperatures and pressures.





Similar content being viewed by others

Notes
Decree of the Government of the Russian Federation dated March 25, 2020 No. 333 “On the adoption by the Russian Federation of amendments to the Montreal Protocol on Substances that Deplete the Ozone Layer.”.
Ghost 34100.3–2017/ISO/IEC Guide 98-3:2008. Measurement Uncertainty. Part 3: Guidance on Expressing Measurement Uncertainty.
References
Thol, M., Lemmon, E.W.: Int J Thermophys 37, 28 (2016). https://doi.org/10.1007/s10765-016-2040-6
Astina, I.M., Budiarso, G., Harrison, R.: Fluid Phase. Equilib. 531, 112921 (2021). https://doi.org/10.1016/j.fluid.2020.112921
Brown, J.S., Di Nicola, G., Zilio, C., Fedele, L., Bobbo, S., Polonara, F.: J. Chem. Eng. Data 57, 3710–3720 (2012). https://doi.org/10.1021/je300945r
Klomfar, J., Souckova, M., Patek, J.: J. Chem. Eng. Data 57, 3270–3277 (2012). https://doi.org/10.1021/je3008974
McLinden, M.J.O., Thol, M., Lemmon, E.W.: Thermodynamic properties of trans‑1,3,3,3-tetrafluoropropene [R1234ze(E)]: measurements of density and vapor pressure and a comprehensive equation of state. Int. Refrig. Air Cond. Conf. 12–15(2189), 1–8 (2010)
Qiu, G., Meng, X., Wu, J.: J. Chem. Thermodyn. 60, 150–158 (2013). https://doi.org/10.1016/j.jct.2013.01.006
Tanaka, K., Takahashi, G., Higashi, Y.: J. Chem. Eng. Data 55, 2169–2172 (2010). https://doi.org/10.1021/je900756g
Tanaka, K., Higashi, Y.: J. Chem. Eng. Data 55, 5164–5168 (2010). https://doi.org/10.1021/je100707s
Yin, J., Zhou, Y., Zhao, G., Ma, S.: Fluid Phase. Equilib, vol. 460., pp. 69–74 (2018). https://doi.org/10.1016/j.fluid.2017.12.030
Zhang, H., Gong, M., Li, H., Guo, H., Dong, X., Wu, J.: Fluid Phase. Equilib, vol. 408., pp. 232–239 (2016). https://doi.org/10.1016/j.fluid.2015.09.010
Wang, L., Song, J., Sheng, B., Dong, X., Zhao, Y., Zhong, Q., Yan, H., Gong, M.: J. Chem. Thermodyn. 141, 105936 (2020). https://doi.org/10.1016/j.jct.2019.105936
Al Ghafri, S.Z.S., Rowland, D., Akhfash, M.: Int. J. Refrig. 98, 249–260 (2019). https://doi.org/10.1016/j.ijrefrig.2018.10.027
Gao, N., Chen, G., Li, R., Wang, Y., He, Y., Yang, B.: J. Therm. Anal. Calorim. 122, 1469–1476 (2015). https://doi.org/10.1007/s10973-015-4837-0
Kagawa, N., Matsuguchi, A., Watanabe, K.: Air Cond. Eng. Trans. Jpn. Soc. Refrig. 28, 71–76 (2011). https://doi.org/10.11322/tjsrae.28.71
Liu, Y., Zhao, X., He, H., Wang, R.: Journal of Chemical & Engineering. Data 63, 113–118 (2018). https://doi.org/10.1021/acs.jced.7b00713
Kano, Y., Kayukawa, Y., Fujii, K.: J. Chem. Eng. Data 58, 2966–2969 (2013). https://doi.org/10.1021/je4004564
Perkins, R.A., McLinden, M.O.: J. Chem. Thermodyn. 91, 43–61 (2015). https://doi.org/10.1016/j.jct.2015.07.005
Chen, Q., Gao, R., Guan, X., Du, L., Chen, G., Tang, L.: J. Chem. Eng. Data 65, 4230–4235 (2020). https://doi.org/10.1021/acs.jced.0c00219
Qi, Y., Yang, H., Zhang, C.: J. Chem. Thermodyn. 135, 68–74 (2019). https://doi.org/10.1016/j.jct.2019.03.016
Yang, T., Hu, X., Meng, X., Wu, J.: J. Chem. Thermodyn. 150, 106222 (2020). https://doi.org/10.1016/j.jct.2020.106222
Gong, M., Zhang, H., Li, H., Zhong, Q., Dong, X., Shen, J., Wu, J.: Int. J. Refrig., 64, 168–175 (2016). https://doi.org/10.10167j.ijrefrig.2016.01.007
Tanaka, K.: J. Chem. Eng. Data 61, 1645–1648 (2016). https://doi.org/10.1021/acs.jced.5b01039
Ye, G., Zh, Y.F.G., Ni, H., Zhuang, Y., Han, X., Chen, G.: J. Chem. Eng. Data 66, 1741–1753 (2021). https://doi.org/10.1021/acs.jced.0c01033
Higashi, Y., Tanaka, K.: J. Chem. Eng. Data 55, 1594–1597 (2010). https://doi.org/10.1021/je900696z
An, B., Yang, F., Yang, K., Duan, Y.: Zh. Yang j. Chem. Eng. Data 63, 2075–2080 (2018). https://doi.org/10.1021/acs.jced.8b00090
Zhang, K., Zh, H.C.Y., Duan, Y.: J. Chem. Thermodyn. 149, 106160 (2020). https://doi.org/10.1016/j.jct.2020.106160
Pierantozzi, M., Tomassetti, S., Di Nicola, G.: Appl. Sci. 10, 2014 (2020). https://doi.org/10.3390/app10062014
Devecioğlu, A.G., Orug, V.: Environ.-Benign Energ Sol. Energ Technol, Green, pp. 87–96 (2020). https://doi.org/10.1007/978-3-030-20637-6_4
Ma, Sh : Modern Theory of Critical Phenomena. Roudedge, vol. 298. New York (1980)
Rykov, S.V., Kudryavtseva, I.V., Phys, J.: Conf. Ser. (2021). https://doi.org/10.1088/1742-6596/2057/1/012112
Rykov, V.A.: J. Eng. Phys. 48, 476–481 (1985). https://doi.org/10.1007/BF00872077
Rykov, S.V., Kudryavtseva, I.V., Rykov, V.A.: J. Phys.: Conf. Ser. (1565). https://doi.org/10.1088/1742-6596/1565/1/012038
Kolobaev, V.A., Rykov, S.V., Kudryavtseva, I.V.: Meas. Techn., 65. No 5, 330–338 (2022). https://doi.org/10.1007/s11018-022-02084-7
Rykov, S.V., Kudryavtseva, I.V., Rykov, V.A., Rykov, S.A., Konyaev, D.V.: Analysis of methods for describing the thermodynamic surface of carbon dioxide. Vestn. Mezhdunar. Akad. Kholoda (2023). https://doi.org/10.17586/1606-4313-2023-22-2-82-88
Rykov, S.V.: The fundamental equation of state considering asymmetry of fluid. Nauchn-tekhnich. Vestn. Povolzh’ya (1), 33–36 (2014). https://elibrary.ru/schqpb
Rykov, V.A., Varfolomeeva, G.B.: J. Eng. Phys. 48, 341–345 (1985). https://doi.org/10.1007/BF00878203
Kozlov, A.D., Lysenkov, V.F., Popov, P.V., Rykov, V.A.: J. Eng. Phys. Thermophys. 62(6), 611–617 (1992). https://doi.org/10.1007/BF00851887
Kolobaev, V.A., Rykov, S.V., Kudryavtseva, I.V., Ustyuzhanin, E.E., Popov, P.V., Rykov, V.A., Kozlov, A.D.: Meas. Techn., 65. No 11, 793–802 (2023). https://doi.org/10.1007/s11018-023-02153-5
Benedek, G.B.: In polarization matie et payonnement, livre de Jubile en l’honneur du proffesor A. Kastler (In French), Presses Universitaires de Paris. Paris (1968)
Rykov, S.V., Kudryavtseva, I.V.: Fund. Res. No 9(8), 1687–1692 (2014)
Widom, B.: J. Chem. Phys. 43, 255–262 (1965). https://doi.org/10.1063/11696618
Kudryavtseva, I.V., Rykov, S.V.: Russ. J. Phys. Chem. A 90, 1493–1495 (2016). https://doi.org/10.1134/S0036024416070153
Rykov, S.V., Sverdlov, A.V., Rykov, V.A., Kudryavtseva, I.V., Ustyuzhanin, E.E.: Method for constructing the equation of state of liquid and gas based on the Migdal phenomenological theory and the Benedek hypothesis. Vestn. Mezhdunar. Akad. Kholoda (2020). https://doi.org/10.17586/1606-4313-2020-19-3-83-90
Bezverkhii, P.P., Dutova, O.S.: Thermophys. Aeromech. 30, 137–151 (2023). https://doi.org/10.1134/S086986432301016X
Rykov, S.V., Kudryavtseva, I.V., Rykov, V.A., Konyaev, D.V.: Saturated vapor pressure of a series of hydrofluoroolefins. Vestn. Mezhdunar. Akad. Kholoda (2022). https://doi.org/10.17586/1606-4313-2022-21-4-76-83
Kolobaev, V.A., Rykov, S.V., Kudryavtseva, I.V., Ustyuzhanin, E.E., Rykov, V.A., Sverdlov, A.V., Kozlov, A.D.: Meas. Techn., 64. No 2, 86–93 (2021). https://doi.org/10.1007/s11018-021-01901-9
Rykov, S.V., Kudryavtseva, I.V., Rykov, V.A., Popov, P.V., Solomichev, R.I., Rykov, S.A.: Description of the thermodynamic surface of argon within the renormalization group theory for an asymmetric model. Vestn. Mezhdunar. Akad. Kholoda (2023). https://doi.org/10.17586/1606-4313-2023-22-3-61-67
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.V. Rykov, P.V. Popov, I.V. Kudryavtseva, and V.A. Rykov declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Translated from Izmeritel’naya Tekhnika, No. 10, pp. 32–40, October, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rykov, S.V., Popov, P.V., Kudryavtseva, I.V. et al. Thermodynamic properties of the trans-1,3,3,3-tetrafluoropropene refrigerant: a method for constructing the equation of state and the tabulated data. Meas Tech 66, 765–775 (2024). https://doi.org/10.1007/s11018-024-02290-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-024-02290-5