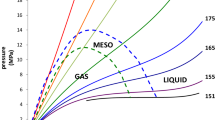
A technique has been developed for constructing a unified fundamental equation of state of an individual substance for a wide range of state parameters. The technique is based on the Benedek hypothesis and the method of pseudocritical points, which are based on the assertion that the isochoric and isobaric heat capacities, the isothermal compressibility coefficient, and the speed of sound in the vicinity of the critical point on the critical and noncritical isochores are described by power-law dependences with the same critical indices. A unified fundamental equation of state of argon has been created that satisfies the requirements of the theory of scaling of critical phenomena, transforms into a virial equation of state in the gas region, satisfactorily conveys experimental data on density, isochoric and isobaric heat capacities, and sound speed within the uncertainty of the initial experimental data in the single-phase region in a wide range temperatures and pressures, on the phase equilibrium line in the range from the triple point to the critical point and in the near-critical region. On the basis of a unified fundamental equation of state, tables of standard reference data for argon were developed and certified in the temperature range of 83.806– 1200 K and pressures of 0.1–1000 MPa, and a statistical estimate of the accuracy of the tables was made.






Similar content being viewed by others
Notes
SDS tables. GSSSD 179-96. Argon liquid and gaseous. Thermodynamic properties, coefficients of dynamic viscosity and thermal conductivity at temperatures of 85 ... 1300 K and pressures of 0.1 ... 1000 MPa.
GOST 34100.3-2017/ISO/IEC Guide 98-3:2008. Measurement uncertainty. Part 3. Guidance on the expression of measurement uncertainty.
GSSSD 396-2022. Argon. Density, enthalpy, isobaric and isochoric heat capacities, entropy and speed of sound in the temperature range from 83.806 K and pressures from 0.1 to 1000 MPa, including the critical region.
References
R. Gilgen, R. Kleinrahm, and W. Wagner, J. Chem. Thermodyn., 26, 383–398 (1994), https://doi.org/10.1006/jcht.1994.1048.
J. Thoen, E. Vangeel, and W. Van Dael, Physica, 45, 339–356 (1969), https://doi.org/10.1016/0031-8914(69)90261-4.
H. M. Roder, R. A. Perkins, and C. A. Nieto de Castro, Int. J. Thermophys., 10, 1141–1164 (1989), https://doi.org/10.1007/BF00500568.
R. A. Perkins, D. G. Friend, H. M. Order, and C. A. Nieto de Castro, Int. J. Thermophys., 12, 965–984 (1991), https://doi.org/10.1007/BF00503513.
J. Klimeck, R. Kleinrahm, and W. Wagner, J. Chem. Thermodyn., 30, 1571–1588 (1998), https://doi.org/10.1006/jcht.1998.0421.
R. Gilgen, R. Kleinrahm, and W. Wagner, J. Chem. Thermodyn., 26, 399–413 (1994), https://doi.org/10.1006/jcht.1994.1049.
A. Michels, Hub Wijker, and H. K. Wijker, Physica, 15, 627–633 (1949), https://doi.org/10.1016/0031-8914(49)90119-6.
A. Michels, J. M. Levelt, and W. de Graaff, Physica, 24, 659–671 (1958), https://doi.org/10.1016/S0031-8914(58)80080-4.
C. Gladun, Cryogenics, 11, 205–209 (1971), https://doi.org/10.1016/0011-2275(71)90312-2.
M. A. Anisimov, B. A. Kovalchuk, V. A. Rabinovich, and V. A. Smirnov, Experimental study of the isochoric heat capacity of argon in a wide range of state parameters, including the critical region, Teplofiz. Svojstva Veshchestv Materialov, 8, 237–245 (1975).
M. A. Anisimov, B. A. Kovalchuk, V. A. Rabinovich, and V. A. Smirnov, Results of an experimental study of the heat capacity Cv of argon in single-phase and two-phase regions, Teplofiz. Svojstva Veshchestv Materialov, 12, 86–106 (1978).
A. V. Voronel', V. C. Gorbunova, V. A. Smirnov, N. G. Shmakov, and V. V. Shchekochikhina, Thermodynamic quantities for pure liquids and the applicability of the asymptotic laws near the critical point, Sov. Phys. JETP, 36, No. 3, 505–513 (1973).
D. H. Bowman, R. A. Aziz, and C. C. Lim, Can. J. Phys., 47, 267–273 (1969).
O. B. Verbeke, V. Jansoone, R. Gielen, and J. De Boelpaep, J. Phys. Chem., 73, 4076–4085 (1969), https://doi.org/10.1021/j100846a008.
W. Wagner, Cryogenics, 13, 470–482 (1973), https://doi.org/10.1016/0011-2275(73)90003-9.
S. N. Biswas, N. J. Trappeniers, P. J. Kortbeek, and C. A. ten Seldam, Rev. Sci. Instrum., 59, 470–476 (1988), https://doi.org/10.1063/1.1139863.
J. Thoen, E. Vangeel, and W. Van Dael, Physica, 52, 205–224 (1971), https://doi.org/10.1016/0031-8914(71)90195-9.
A. F. Estrada-Alexanders and J. P. M. Trusler, Int. J. Thermophys., 17, 1325–1347 (1996), https://doi.org/10.1007/BF01438673.
E. C. Morris and R. G. Wylie, J. Chem. Phys., 73, 1359–1367 (1980), https://doi.org/10.1063/1.440252.
S. L. Robertson, S. E. Babb, and G. J. Scott, J. Chem. Phys., 50, 2160–2166 (1969), https://doi.org/10.1063/1.1671345.
A. Lecocq, "Determination Experimentale des Equations d'etat de L'Argon jusqua 1000°C et 1000 kg/cm2," J. Recherches Centre National Recherche Scientific, 55, 55–82 (1960). (In Fr.)
M. Baba, L. Dordain, Y.-Y. Coxam, and J.-P. Grolier, "Calorimetric measurements of heat capacities and heats of mixing in the range 300–570 K and up to 30 MPa," Indian J. Technol., 30, 553–558 (1992).
A. F. Estrada-Alexanders and J. P. M. Trusler, J. Chem. Thermodyn., 27, 1075–1089 (1995), https://doi.org/10.1006/jcht.1995.0113.
M. B. Ewing and J. P. M. Trusler., Physica A, 184, 415–436 (1992), https://doi.org/10.1016/0378-4371(92)90314-G.
M. B. Ewing and A. R. H. Goodwin, J. Chem. Thermodyn., 24, 531–547 (1992), https://doi.org/10.1016/S0021-9614(05)80123-5.
M. B. Ewing, A. A. Owusu, and J. P. M. Trusler, Physica A, 156, 899–908 (1989), https://doi.org/10.1016/0378-4371(89)90026-5.
S. S. Lestz, J. Chem. Phys., 38, 2830–2834 (1963), https://doi.org/10.1063/1.1733609.
C. Tegeler, R. Span, and W. Wagner, J. Phys. Chem. Ref. Data, 28, 779–849 (1999), https://doi.org/10.1063/1.556037.
R. B. Stewart and R. T. Jacobsen, J. Phys. Chem. Ref. Data, 18, 639–799 (1989), https://doi.org/10.1063/1.555829.
A. Rizi and A. Abbaci, J. Mol. Liq., 171, 64–70 (2012), https://doi.org/10.1016/j.molliq.2012.04.010.
S. V. Rykov, I. V. Kudryavtseva, and V. A. Rykov, J. Phys.: Conf. Ser., 1385, 012014 (2019), https://doi.org/10.1088/1742-6596/1385/1/012014.
V. A. Rykov, J. Eng. Phys. Thermophys., 48, No. 4, 476–481 (1995), https://doi.org/10.1007/BF00872077.
V. A. Rykov, S. V. Rykov, I. V. Kudryavtseva, and A. V. Sverdlov, J. Phys.: Conf. Ser., 891, 012334 (2017), https://doi.org/10.1088/1742-6596/891/1/012334.
G. B. Benedek, Polarisation Matiere et Rayonnement — Volume Jubilaire en l'Honneur d'Alfred Kastler, Presses Uni- versitaires de Paris, Paris (1968), p. 71.
I. V. Kudryavtseva, V. A. Rykov, S. V., Rykov, and E. E. Ustyuzhanin, J. Phys. Conf. Ser., 946, 012118 (2018), https://doi.org/10.1088/1742-6596/946/1/012118.
A. D. Kozlov, V. F. Lysenkov, P. V. Popov, and V. A. Rykov, J. Eng. Phys. Thermophys., 62, 611–617 (1992), https://doi.org/10.1007/BF00851887.
I. V. Kudryavtseva and S. V. Rykov, Russ. J. Phys. Chem. A, 90, No. 7, 1493–1495 (2016), https://doi.org/10.1134/S0036024416070153.
S. V. Rykov, I. V. Kudryavtseva, V. A. Rykov, and E. E. Ustyuzhanin, J. Phys. Conf. Ser., 2057, 012113 (2021), https://doi.org/10.1088/1742-6596/2057/1/012113.
V. A. Kolobaev, S. V. Rykov, I. V. Kudryavtseva, et al., Meas. Tech., 64, No. 2, 86–93 (2021), https://doi.org/10.1007/s11018-021-01901-9.
V. P. Skripov, E. N. Sinicin, P. A. Pavlov, et. al., Teplofizicheskie svojstva zhidkostej v metastabil'nom sostoyanii, Atomizdat Publ., Moscow (1980), 208 p.
V. A. Kolobaev, S. V. Rykov, I. V. Kudryavtseva, et al., Thermodynamic properties of R1233zd(E) refrigerant: a technique for constructing the fundamental equation of state and tabulated data, Izmerit. Tekh., No. 5, 22–28 (2022), https://doi.org/10.32446/0368-1025it.2022-5-22-28.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel'naya Tekhnika, No. 11, pp. 9–16, October, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolobaev, V.A., Rykov, S.V., Kudryavtseva, I.V. et al. Unified Fundamental Equation of State of Argon: Construction Technique Within the Framework of Scaling Theory and Tables of Standard Reference Data. Meas Tech 65, 793–802 (2023). https://doi.org/10.1007/s11018-023-02153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-023-02153-5