Abstract
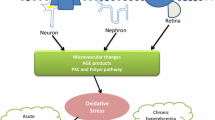
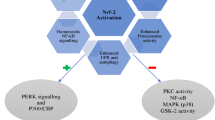
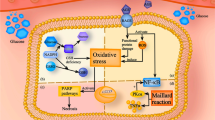
About 50% of the diabetic patients worldwide suffer from Diabetic peripheral neuropathy (DPN) which is characterized by chronic pain and loss of sensation, frequent foot ulcerations, and risk for amputation. Numerous factors like hyperglycemia, oxidative stress (OS), impaired glucose signaling, inflammatory responses, neuronal cell death are known to be the various mechanisms underlying DACD and DPN. Development of tolerance, insufficient and inadequate relief and potential toxicity of classical antinociceptives still remains a challenge in the clinical setting. Therefore, there is an emerging need for novel treatments which are both without any potential side effects as well as which focus more on the pathophysiological mechanisms underlying the disease. Also, sirtuins are known to deacetylate Nrf2 and contribute to its action of reducing ROS by generation of anti-oxidant enzymes. Therefore, targeting sirtuins could be a favourable therapeutic strategy to treat diabetic neuropathy by reducing ROS and thereby alleviating OS in DPN. In the present review, we outline the potential use of SIRT1 activators as therapeutic alternatives in treating DPN. We have tried to highlight how sirtuins are interlinked with Nrf2 and NF-κB and put forth how SIRT activators could serve as potential therapy for DPN.



Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Aruoma OI, Halliwell B, Butler J, Hoey BM (1989) Apparent inactivation of alpha 1-antiproteinase by sulphur-containing radicals derived from penicillamine. Biochem Pharmacol 38(24):4353–4357
Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL (2011) SIRT3 and cancer: Tumor promoter or suppressor? Biochim Biophys Acta - Rev Cancer 1816(1):80–88
Azmi S, Petropoulos IN, Ferdousi M, Ponirakis G, Alam U, Malik RA (2019). An update on the diagnosis and treatment of diabetic somatic and autonomic neuropathy. F1000Research;8.
Bae NS, Swanson MJ, Vassilev A, Howard BH (2004) Human histone deacetylase SIRT2 interacts with the homeobox transcription factor HOXA10. J Biochem (tokyo) 135:695–700
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120(4):483–495
Basta G, Schmidt AM, De Caterina R (2004) Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res 63(4):582–592
Bennett MI, Simpson KH (2004) Gabapentin in the treatment of neuropathic pain. Palliat Med 18(1):5–11
Bheda P, Jing H, Wolberger C, Lin H (2016) The substrate specificity of sirtuins. Annu Rev Biochem 85:405–429
Bheereddy P, Yerra VG, Kalvala AK, Sherkhane B, Kumar A (2020) SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell Mol Neurobiol 18:1–5
Brownlee M (2001) biology of diabetic complications. 414(December):813–20
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Calcutt NA, Tomlinson DR, Biswas S (1990) Coexistence of nerve conduction deficit with increased Na(+)-K(+)-ATPase activity in galactose-fed mice. Implications for polyol pathway and diabetic neuropathy. Diabetes. 39(6):663–6.
Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27:2276–2288
Caton PW, Richardson SJ, Kieswich J, Bugliani M, Holland ML, Marchetti P et al (2013) Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia 56:1068–1077
Chandrasekaran K, Salimian M, Konduru SR, Choi J, Kumar P, Long A, Klimova N, Ho CY, Kristian T, Russell JW (2019) Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain 142(12):3737–3752
Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE (1998) Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem 273(14):8225–8231
Chen B, Zang W, Wang J, Huang Y, He Y, Yan L et al (2015) The chemical biology of sirtuins. Chem Soc Rev [internet] 44(15):5246–5264
Chiurchiù V, MacCarrone M (2011) Chronic inflammatory disorders and their redox control: From molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal 15(9):2605–2641
Cicero AFG, Baggioni A (2016) Berberine and its role in chronic disease. Adv Exp Med Biol 928:27–45
Cohen K, Shinkazh N, Frank J, Israel I, Fellner C (2015) Pharmacological treatment of diabetic peripheral neuropathy. Pharmacy Ther 40(6):372
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC et al (2009) RAGE induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20(4):742–752
Cui X, Yao L, Yang X, Gao Y, Fang F, Zhang J et al (2017) SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK. Am J Physiol Endocrinol Metab 313:E493–E505
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 324 ( Pt 1(Pt 1):1–18.
Donmez G (2012) The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33(9):494–501
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5(3):344–352
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F et al (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97(22):12222–12226
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C et al (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112(7):1049–1057
Du J et al (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334:806–819
Feng X, Koh DW (2013) Inhibition of poly(ADP-ribose) polymerase-1 or poly(ADP-ribose) glycohydrolase individually, but not in combination, leads to improved chemotherapeutic efficacy in HeLa cells. Int J Oncol 42(2):749–756
Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP et al (2011) Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 6:e23295
Ganesh Yerra V, Negi G, Sharma SS, Kumar A (2013) Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol [internet] 1(1):394–397
Geraldes P, King GL (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106(8):1319–1331
Ghafourifar P, Bringold U, Klein SD, Richter C (2001) Mitochondrial nitric oxide synthase, oxidative stress and apoptosis. Biol Signals Recept 10(1–2):57–65
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Guarente L, Franklin H (2011) Epstein lecture: sirtuins, aging, and medicine. N Engl J Med 364:2235–2244
Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, Donofrio P, Cornblath D, Sachdeo R, Siu CO, Kamin M (1998) Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 50(6):1842–1846
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125
Imai SI, Guarente L (2010) Ten years of NAD-dependent SIR2 family deacetylases – implications for metabolic diseases. Trends Pharm Sci 31:212–220
Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A (2015) Islet betacell failure in type 2 diabetes–Within the network of toxic lipids. Biochem Biophys Res Commun 460:491–496
Jing H, Lin H (2015) Sirtuins in epigenetic regulation. Chem Rev 115(6):2350–2375
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D et al (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA 108:14608–14613
Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L et al (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483:218–221
Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaBdependent gene expression and organismal life span. Cell 136:62–74
Kim M, Lee JS, Oh JE, Nan J, Lee H, Jung HS et al (2015) SIRT3 overexpression attenuates palmitate-induced pancreatic beta-cell dysfunction. PLoS ONE. 10:e0124744
Kochar DK, Rawat N, Agrawal RP, Vyas A, Beniwal R, Kochar SK, Garg P (2004) Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM 97(1):33–38
Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED (1998) High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest 101(1):160–169
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Koya D, King GL (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47(6):859–866
Kuchibhotla P, Rao BD (1995) A methodology for fast scheduling of partitioned systolic algorithms. J VLSI Signal Process 10(2):111–126
Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM (1997) Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem 272(28):17810–17814
Lee HB, Yu MR, Song JS, Ha H (2004) Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int 65(4):1170–1179
Lemos V, de Oliveira RM, Naia L, Szegö É, Ramos E, Pinho S et al (2017) The NAD+-dependent deacetylase SIRT2 attenuates oxidative stress and mitochondrial dysfunction and improves insulin sensitivity in hepatocytes. Hum Mol Genet 26:4105–4117
Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci 27:2606–2616. https://doi.org/10.1523/JNEUROSCI.3694-07.2007
Lithner F (2000) Venlafaxine in treatment of severe painful peripheral diabetic neuropathy. Diabetes Care. 23(11):1710
Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE (1993) 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health 40(2–3):391–404
Lu X, Zhang L, Li P et al (2017) The protective effects of compatibility of Aconiti Lateralis Radix Praeparata and Zingiberis Rhizoma on rats with heart failure by enhancing mitochondrial biogenesis via Sirt1/PGC-1α pathway. Biomed Pharmacother 92:651–660
Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I (2005) Evolutionarily Conserved and Nonconserved Cellular Localizations and Functions of Human SIRT Proteins. Mol Biol Cell
Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M et al (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452:492–496
Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O et al (2009) Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle 8:2664–2666
Morrison AD, Clements RSJ, Travis SB, Oski F, Winegrad AI (1970) Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun 40(1):199–205
Nakagawa T, Guarente L (2011) Sirtuins at a glance. J Cell Sci 124(6):833–838
Negi G, Kumar A, Joshi RP, Sharma SS (2011) Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun [internet] 408(1):1–5
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11(2):437–444
Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P et al (2004) Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes 53(3):711–720
Ogura M, Nakamura Y, Tanaka D, Zhuang X, Fujita Y, Obara A, Hamasaki A, Hosokawa M, Inagaki N (2010) Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem Biophys Res Commun 393(1):73–78
Ogura Y, Kitada M, Monno I, Kanasaki K, Watanabe A, Koya D (2018) Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep 23:153–159
Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B (2015) Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015
Oza MJ, Kulkarni YA (2020) Formononetin ameliorates diabetic neuropathy by increasing expression of SIRT1 and NGF. Chem Biodivers. 17(6):e2000162
Pacher P, Obrosova IG, Mabley JG, Szabó C (2005) Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 12(3):267–275
Pacher P, Beckman JS, Liaudet L. {suggests cell models with genetic deletion of iNOS as control experiment. Can we do this too?] Nitric oxide and peroxynitrite in health and disease. Physiol Rev [Internet]. 2007;87(1):315–424.
Pan H, Guan D, Liu X, Li J, Wang L, Wu J et al (2016) SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res 26:190–205
Park Y (2014) Oxidative Stress and Diabetic Neuropathy. 2014;3–13.
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 105(28):9793–9798
Pop-Busui R, Sima A, Stevens M (2006) Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev 22(4):257–273
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12:662–667
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288(2):481–487
Rine J, Herskowitz I (1987) Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 116:9–22
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123:4251–4258. https://doi.org/10.1242/jcs.073783
Sadi Gk, Konat D (2016) Resveratrol regulates oxidative biomarkers and antioxidant enzymes in the brain of streptozotocininduced diabetic rats. Pharm Biol 54:1156–1163
Saeed T, Nasrullah M, Ghafoor A, Shahid R, Islam N, Khattak MU, Maheshwary N, Siddiqi A, Khan MA (2014) Efficacy and tolerability of carbamazepine for the treatment of painful diabetic neuropathy in adults: a 12-week, open-label, multicenter study. Int J Gen Med 7:339
Sanchez-Fidalgo S, Villegas I, Sanchez-Hidalgo M, Alarcon de la Lastra C (2012) Sirtuin modulators: mechanisms and potential clinical implications. Curr Med Chem 9(15):2414–2441
Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int J Endocrinol. 2014(July).
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30(11):1191–1212
Schleicher ED, Weigert C (2000) Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int Suppl. 58(77).
Shakeel M (2015) Recent advances in understanding the role of oxidative stress in diabetic neuropathy. Diabetes Metab Syndr Clin Res Rev [internet] 9(4):373–378
Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, MacK NJ, Ahmad N (2018) The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxidants Redox Signal 28(8):643–661
Singh V, Ubaid S (2020) Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 43(5):1589–1598
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C et al (2010) Sirt3 mediates reduction of oxidative damage and prevention of agerelated hearing loss under caloric restriction. Cell 143:802–812
Sun LQ, Zhao J, Zhang TT et al (2012) Protective effects of salvianolic acid B on Schwann cells apoptosis induced by high glucose. Neurochem Res 37:996–1010
Tang WH, Martin KA, Hwa J (2012) Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol 3(May):1–8
Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Gene Dev 20(10):1256–1261
Vaulont S, Vasseur-Cognet M, Kahn A (2000) Glucose regulation of gene transcription. J Biol Chem [internet] 275(41):31555–31558
Vadivelu N, Kai A, Maslin B, Kodumudi G, Legler A, Berger JM (2015) Tapentadol extended release in the management of peripheral diabetic neuropathic pain. Ther Clin Risk Manag 11:95
Villalba JM, Alcaín FJ (2012) Sirtuin activators and inhibitors. Biofactors 38(5):349–359
Vinik A, Rosenstock J, Sharma U, Feins K, Hsu C, Merante D (2014) Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-and active comparator–controlled, adaptive proof-of-concept phase 2 study. Diabetes Care 37(12):3253–3261
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6:505–514. https://doi.org/10.1111/j.1474-9726.2007.00304.x
Wang H, Qiang L, Farmer SR (2008) Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 28:188–200
Wang BB, Wang JL, Yuan J et al (2016) Sugar composition analysis of Fuzi Polysaccharides by HPLC-MSn and their protective effects on Schwann cells exposed to high glucose. Molecules 21:1496
Wang W, Sun W, Cheng Y, Xu Z, Cai L (2019) Role of sirtuin-1 in diabetic nephropathy. 291–309.
Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL (2001) Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280(5):E685–E694
Xiong X, Wang G, Tao R, Wu P, Kono T, Li K et al (2016) Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia 59:151–160
Yerra VG, Kalvala AK, Kumar A (2017) Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J Nutr Biochem 47:41–52
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380
Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ et al (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297(5579):259–263
Yu X, Zhang L, Yang X et al (2012) Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis. Molecules 17:11216–11228
Zhang Q, Liang XC (2019) Effects of mitochondrial dysfunction via AMPK/PGC-1 α signal pathway on pathogenic mechanism of diabetic peripheral neuropathy and the protective effects of Chinese medicine. Chin J Integr Med 25(5):386–394
Zhang Z, Ding X, Zhou Z, Qiu Z, Shi N, Zhou S, Du L, Zhu X, Wu Y, Yin X, Zhou C (2019) Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain 160(5):1082–1092
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327:1000–1004
Zhou Y, Chung ACK, Fan R, Lee HM, Xu G, Tomlinson B et al (2017) Sirt3 deficiency increased the vulnerability of pancreatic beta cells to oxidative stress-induced dysfunction. Antioxid Redox Signal 27:962–976
Author information
Authors and Affiliations
Contributions
Conceptualization and literature search- Shivangi Patel, Literature search and data analysis-Hasnat Khan, Critical revision of the work-Anuradha Majumdar.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, S., Khan, H. & Majumdar, A. Crosstalk between Sirtuins and Nrf2: SIRT1 activators as emerging treatment for diabetic neuropathy. Metab Brain Dis 37, 2181–2195 (2022). https://doi.org/10.1007/s11011-022-00956-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-00956-z