Abstract
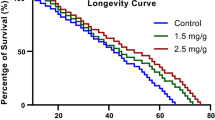
African eggplant (Solanum macrocarpon L) (AE) and Black Nightshade (Solanum nigrum L) (BN) leaves are green leafy vegetables with nutritional and ethnobotanical values. We have previously characterized the vegetables via HPLC/LC-MS to reveal notable phenolic acids, flavonoids and alkaloids. In this present study, we addressed the efficacy of the two vegetables in mitigating mercuric chloride (HgCl2)-induced neurotoxicity and memory impairment in Drosophila melanogaster. Flies were exposed to HgCl2 (0.30 mg/g) alone or in combination with the vegetables (0.1 and 1.0%) of both samples in their diets for seven days. The results showed that HgCl2 (Hg)-exposed flies had significantly reduced survival rate and memory index, which were ameliorated in the Hg-exposed flies fed AE or BN. This was accompanied by increased reactive oxygen species (ROS) levels, reduced total thiol, as well as catalase, glutathione transferase (GST) and acetylcholine esterase (AChE) activities in Hg-exposed fly heads, but ameliorated in Hg-exposed flies fed dietary inclusions of the vegetables. In addition, the Hg-induced alterations in SOD, NF-ҝB/Relish, Dronc and Reaper mRNA levels were statistically indistinguishable from controls in Hg-treated flies fed diets containing AE or BN. Normalization of cnc/Nrf2 and FOXO were observed only in Hg-treated flies fed BN. These findings suggest that dietary AE or BN leaves offer protection against Hg-induced memory impairment and neurotoxicity in D. melanogaster, and further justify them as functional foods with neuroprotective properties.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Abdel Moneim AE (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis. https://doi.org/10.1007/s11011-015-9652-6
Abolaji A, Kamdem JP, Farombi O, et al (2013) Drosophila melanogaster as a Promising Model Organism in Toxicological Studies: A Mini Review Ameliorative Properties of Citrus Bioflavonoids on 4-vinylcyclohexene diepoxide (VCD)-induced reprotoxicity View project Nigerian Drosophila Research Community V. Arch Basic Appl Med 1 33 - 38 1:33–38
Adedara IA, Rosemberg DB, Souza DO et al (2015) Biochemical and behavioral deficits in the lobster cockroach Nauphoeta cinerea model of methylmercury exposure. Toxicol Res (Camb). https://doi.org/10.1039/c4tx00231h
Adedara IA, Abolaji AO, Rocha JBT, Farombi EO (2016) Diphenyl Diselenide protects against mortality, locomotor deficits and oxidative stress in Drosophila melanogaster model of manganese-induced neurotoxicity. Neurochem Res https://doi.org/10.1007/s11064-016-1852-x
Aebi H (1984) [13] Catalase in Vitro. Methods Enzymol. https://doi.org/10.1016/S0076-6879(84)05016-3
Afolabi BA, Olagoke OC (2020) High concentration of MSG alters antioxidant defence system in lobster cockroach Nauphoeta cinerea (Blattodea: Blaberidae). BMC Res Notes 13:217. https://doi.org/10.1186/s13104-020-05056-8
Afolabi BA, Olagoke OC, Souza DO et al (2020) Modified expression of antioxidant genes in lobster cockroach, Nauphoeta cinerea exposed to methylmercury and monosodium glutamate. Chem Biol Interact 318:108969. https://doi.org/10.1016/j.cbi.2020.108969
Ajiboye BO, Akalabu MC, Ojo OA et al (2018) Inhibitory effect of ethyl acetate fraction of Solanum macrocarpon L. leaves on cholinergic, monoaminergic, and purinergic enzyme activities. J Food Biochem. https://doi.org/10.1111/jfbc.12643
Akinyemi AJ, Oboh G, Ogunsuyi O, et al (2018) Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in Drosophila melanogaster model. Metab Brain Dis 33:. https://doi.org/10.1007/s11011-017-0100-7
Anyanwu BO, Orish CN, Ezejiofor AN et al (2020) Neuroprotective effect of Costus afer on low dose heavy metal mixture (lead, cadmium and mercury) induced neurotoxicity via antioxidant, anti-inflammatory activities. Toxicol Reports. https://doi.org/10.1016/j.toxrep.2020.08.008
Aragão WAB, Teixeira FB, Fagundes NCF et al (2018) Hippocampal dysfunction provoked by mercury chloride exposure: evaluation of cognitive impairment, oxidative stress, tissue injury and nature of cell death. Oxidative Med Cell Longev. https://doi.org/10.1155/2018/7878050
Aschner M, Culbreth M (2018) GSK-3β, a double-edged sword in Nrf2 regulation: implications for neurological dysfunction and disease [version 1; referees: 2 approved]. F1000Research
Beg T, Jyoti S, Naz F et al (2018) Protective effect of Kaempferol on the transgenic Drosophila model of Alzheimer’s disease. CNS Neurol Disord - Drug Targets. https://doi.org/10.2174/1871527317666180508123050
Bianchini MC, Gularte COA, Nogara PA et al (2019) Thimerosal inhibits Drosophila melanogaster tyrosine hydroxylase (DmTyrH) leading to changes in dopamine levels and impaired motor behavior: implications for neurotoxicity. Metallomics. https://doi.org/10.1039/c8mt00268a
Biteau B, Karpac J, Hwangbo DS, Jasper H (2011) Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol 46:349–354. https://doi.org/10.1016/j.exger.2010.11.003
Bjørklund G, Tinkov AA, Dadar M, et al (2019) Insights into the potential role of mercury in Alzheimer’s disease. J Mol Neurosci 67(4)511–533
Branco V, Carvalho C (2019) The thioredoxin system as a target for mercury compounds. Biochim Biophys Acta - Gen Subj 1863(12)129255
Branco V, Godinho-Santos A, Gonçalves J et al (2014) Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2014.04.030
Cashio P, Lee TV, Bergmann A (2005) Genetic control of programmed cell death in Drosophila melanogaster. Semin Cell Dev Biol 16:225–235. https://doi.org/10.1016/j.semcdb.2005.01.002
Culbreth M, Zhang Z, Aschner M (2017) Methylmercury augments Nrf2 activity by downregulation of the Src family kinase Fyn. Neurotoxicology. https://doi.org/10.1016/j.neuro.2017.07.028
De La Lastra CA, Villegas I (2005) Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 49(5)405–430
El Asar HM, Mohammed EA, Aboulhoda BE et al (2019) Selenium protection against mercury neurotoxicity: modulation of apoptosis and autophagy in the anterior pituitary. Life Sci. https://doi.org/10.1016/j.lfs.2019.116578
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. https://doi.org/10.1016/0006-2952(61)90145-9
Farina M, Franco JL, Ribas CM et al (2010) Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice†. J Pharm Pharmacol. https://doi.org/10.1211/jpp.57.11.0017
Ghavami S, Shojaei S, Yeganeh B, et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Ghosh S, May MJ, Kopp EB (1998) NF-κB and rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260. https://doi.org/10.1146/annurev.immunol.16.1.225
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 49(22):7130–7139
Hazelhoff MH, Bulacio RP, Torres AM (2012) Gender related differences in kidney injury induced by mercury. Int J Mol Sci. https://doi.org/10.3390/ijms130810523
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res
Johnson O, Becnel J, Nichols CD (2011) Serotonin receptor activity is necessary for olfactory learning and memory in Drosophila melanogaster. Neuroscience. https://doi.org/10.1016/j.neuroscience.2011.06.058
Ki WL, Kundu JK, Sue OK et al (2006) Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-κB and MAPKs in mouse skin in vivo. J Nutr. https://doi.org/10.1093/jn/136.5.1150
Lashmanova E, Proshkina E, Zhikrivetskaya S et al (2015) Fucoxanthin increases lifespan of Drosophila melanogaster and Caenorhabditis elegans. Pharmacol Res. https://doi.org/10.1016/j.phrs.2015.08.009
Lee YW, Ha MS, Kim YK (2001) Role of reactive oxygen species and glutathione in inorganic mercury-induced injury in human glioma cells. Neurochem Res. https://doi.org/10.1023/A:1013955020515
Lee KS, Iijima-Ando K, Iijima K et al (2009) JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J Biol Chem 284:29454–29461. https://doi.org/10.1074/jbc.M109.028027
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. https://doi.org/10.1006/meth.2001.1262
Maitra U, Harding T, Liang Q, Ciesla L (2020) GardeninA confers neuroprotection against environmental toxin in a. bioRxiv
Mary OO, Sebastine OU, Ejuiwa MC, et al (2020) Anxiolytic and curative effect of Solanum macrocarpon leaves extract on acetaminophen induced brain injury in adult Wistar rats 9:205–212
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1(2):120–130
Monnet-Tschudi F, Zurich MG, Boschat C, et al (2006) Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health
Moretto MB, Lermen CL, Morsch VM et al (2004) Effect of subchronic treatment with mercury chloride on NTPDase, 5′-nucleotidase and acetylcholinesterase from cerebral cortex of rats. J Trace Elem Med Biol. https://doi.org/10.1016/S0946-672X(04)80027-0
Morey M, Corominas M, Serras F (2003) DIAP1 suppresses ROS-induced apoptosis caused by impairment of the selD/sps1 homolog in Drosophila. J Cell Sci
Ni M, Li X, Yin Z et al (2010) Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci. https://doi.org/10.1093/toxsci/kfq126
Nogara PA, Oliveira CS, Schmitz GL, et al (2019) Methylmercury’s chemistry: from the environment to the mammalian brain. Biochim Biophys Acta - Gen Subj 1863(12):129284
Ogunsuyi OB, Ademiluyi AO, Oboh G et al (2018) Green leafy vegetables from two Solanum spp. (Solanum nigrum L and Solanum macrocarpon L) ameliorate scopolamine-induced cognitive and neurochemical impairments in rats. Food Sci Nutr 6. https://doi.org/10.1002/fsn3.628
Ogunsuyi OB, Ademiluyi AO, Oboh G (2020a) Solanum leaves extracts exhibit antioxidant properties and inhibit monoamine oxidase and acetylcholinesterase activities (in vitro) in Drosophila melanogaster. J Basic Clin Physiol Pharmacol 31. https://doi.org/10.1515/jbcpp-2019-0256
Ogunsuyi OB, Oboh G, Özek G, Göger F (2020b) Solanum vegetable-based diets improve impairments in memory, redox imbalance, and altered critical enzyme activities in Drosophila melanogaster model of neurodegeneration. J Food Biochem. https://doi.org/10.1111/jfbc.13150
Ogunsuyi OB, Olagoke OC, Afolabi BA et al (2021) Dietary inclusions of Solanum vegetables mitigate aluminum-induced redox and inflammation-related neurotoxicity in Drosophila melanogaster model. Nutr Neurosci 0:1–15. https://doi.org/10.1080/1028415x.2021.1933331
Okesola MA, Ajiboye BO, Oyinloye BE et al (2020) Effect of Solanum macrocarpon Linn leaf aqueous extract on the brain of an alloxan-induced rat model of diabetes. J Int Med Res. https://doi.org/10.1177/0300060520922649
Olagoke OC, Segatto AL, Afolabi BA, Rocha JBT (2021) Streptozotocin induces brain glucose metabolic changes and alters glucose transporter expression in the lobster cockroach; Nauphoeta cinerea (Blattodea: Blaberidae). Mol Cell Biochem. https://doi.org/10.1007/s11010-020-03976-4
Oliveira CS, Piccoli BC, Aschner M, Rocha JBT (2017) Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In: Advances in Neurobiology Neurotoxicity of metals 53–83
Paula MT, Zemolin AP, Vargas AP, et al (2014) Effects of Hg(II) exposure on MAPK phosphorylation and antioxidant system in D. melanogaster. Environ Toxicol. https://doi.org/10.1002/tox.21788
Pérez-Severiano F, Santamaría A, Pedraza-Chaverri J et al (2004) Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in Striata of mice transgenic for the Huntington’s disease mutation. Neurochem Res. https://doi.org/10.1023/B:NERE.0000018843.83770.4b
Periyanayagam K, Gokila S, Balasubramaniam KG et al (2015) Protective effect of the leaves of Solanum torvums wartz on Drosophila melanogaster against ß-amyloid induced alzheimer disease. Res J Pharm Technol. https://doi.org/10.5958/0974-360X.2015.00114.6
Piccoli BC, Segatto ALA, Oliveira CS et al (2019) Simultaneous exposure to vinylcyclohexene and methylmercury in Drosophila melanogaster: biochemical and molecular analyses. BMC Pharmacol Toxicol 20:1–17. https://doi.org/10.1186/s40360-019-0356-0
Piccoli BC, Alvim JC, da Silva FD et al (2020) High level of methylmercury exposure causes persisted toxicity in Nauphoeta cinerea. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06989-9
Pinho AI, Oliveira CS, Lovato FL et al (2017) Antioxidant and mercury chelating activity of Psidium guajava var. pomifera L. leaves hydroalcoholic extract. J Toxicol environ heal - part a Curr issues. https://doi.org/10.1080/15287394.2017.1382408
Pitoniak A, Bohmann D (2015) Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic Biol Med 88:302–313
Radyuk SN, Sohal RS, Orr WC (2003) Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors: over- and under-expression of thioredoxin peroxidase in Drosophila cells. Biochem J 371:743–752. https://doi.org/10.1042/BJ20021522
Showell SS, Martinez Y, Gondolfo S et al (2020) Overexpression of the vesicular acetylcholine transporter disrupts cognitive performance and causes age-dependent locomotion decline in Drosophila. Mol Cell Neurosci. https://doi.org/10.1016/j.mcn.2020.103483
Sumathi T, Shobana C, Christinal J, Anusha C (2012) Protective effect of Bacopa monniera on methyl mercury-induced oxidative stress in cerebellum of rats. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-012-9813-7
Sykiotis GP, Bohmann D (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. https://doi.org/10.1016/j.devcel.2007.12.002
Wang L, Jiang H, Yin Z et al (2009) Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol Sci. https://doi.org/10.1093/toxsci/kfn201
Yang B, Yin C, Zhou Y et al (2019) Curcumin protects against methylmercury-induced cytotoxicity in primary rat astrocytes by activating the Nrf2/ARE pathway independently of PKCδ. Toxicology. https://doi.org/10.1016/j.tox.2019.152248
Zaidi SK, Hoda MN, Tabrez S et al (2014) Protective effect of Solanum nigrum leaves extract on immobilization stress induced changes in rat’s brain. Evidence-based Complement Altern Med. https://doi.org/10.1155/2014/912450
Zamberlan DC, Halmenschelager PT, Silva LFO, da Rocha JBT (2020a) Copper decreases associative learning and memory in Drosophila melanogaster. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.135306
Zamberlan DC, Halmenschelager PT, Silva LFO, da Rocha JBT (2020b) Measured data of Drosophila melanogaster (Diptera Drosophilidae) development and learning and memory behaviour after copper exposition. Data Br. https://doi.org/10.1016/j.dib.2019.104986
Zars T, Fischer M, Schulz R, Heisenberg M (2000) Localization of a short-term memory in Drosophila. Science (80-). https://doi.org/10.1126/science.288.5466.672
Zhang J, Zhang X, Wen C et al (2019) Lotus seedpod proanthocyanidins protect against neurotoxicity after methyl-mercuric chloride injury. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2019.109560
Zhu J, Wang C, Gao X et al (2019) Comparative effects of mercury chloride and methylmercury exposure on early neurodevelopment in zebrafish larvae. RSC Adv. https://doi.org/10.1039/c9ra00770a
Acknowledgements
Ogunsuyi B. Opeyemi is a recipient of the 2018 CNPq-TWAS Sandwich Postgraduate Fellowship (FR number: 314765/2018-2). Olawande C. Olagoke is a recipient of the 2017 CNPq-TWAS Postgraduate Fellowship (FR number: 3240299312). The financial support of CAPES, FAPERGS (DocFix) and CNPq is also acknowledged.
Author information
Authors and Affiliations
Contributions
OBO was involved in conceptualizing the research and carried out the data collection and analysis as well as manuscript drafting. OCO was involved in collection, analysis and interpretation of data regarding the RT-qPCR analysis. BAA was involved in collection of data regarding the RT-qPCR analysis. JSL was involved in collection of data regarding the assessment of fly memory function. AOA was involved in data analysis and research supervision. MA was involved in data analysis and interpretation, as well as correction and approval of final draft of manuscript. GO was involved in research conceptualization and supervision. NVB was involved in fly culture and data collection. JBTR was involved in research conceptualization and supervision, data interpretation, as well as writing, correction and approval of final draft of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 195 kb)
Rights and permissions
About this article
Cite this article
Ogunsuyi, O.B., Olagoke, O.C., Afolabi, B.A. et al. Effect of Solanum vegetables on memory index, redox status, and expressions of critical neural genes in Drosophila melanogaster model of memory impairment. Metab Brain Dis 37, 729–741 (2022). https://doi.org/10.1007/s11011-021-00871-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00871-9