Abstract
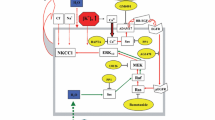
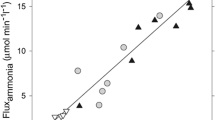
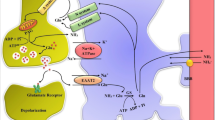
Ion channels, exchangers and transporters are known to be involved in cell volume regulation. A disturbance in one or more of these systems may result in loss of ion homeostasis and cell swelling. In particular, activation of the Na+–K+–Cl− cotransporters has been shown to regulate cell volume in many conditions. The Na+–K+–Cl− cotransporters (NKCC) are a class of membrane proteins that transport Na, K, and Cl ions into and out of a wide variety of epithelial and nonepithelial cells. Studies have established the role of NKCC1 in astrocyte swelling/brain edema in ischemia and trauma. Our recent studies suggest that NKCC1 activation is also involved in astrocyte swelling induced by ammonia and in the brain edema in the thioacetamide model of acute liver failure. This review will focus on mechanisms of NKCC1 activation and its contribution to astrocyte swelling/brain edema in neurological disorders, with particular emphasis on ammonia neurotoxicity and acute liver failure.

Similar content being viewed by others
Abbreviations
- ALF:
-
acute liver failure
- CaMKII:
-
calcium/calmodulin-dependent protein kinases II
- cAMP:
-
Cyclic adenosine monophosphate
- CHX:
-
cyclohexamide
- H2O2 :
-
hydrogen peroxide
- L-NAME:
-
L-NG-Nitroarginine methyl ester
- MAPKs:
-
mitogen-activated protein kinases
- NCCa-ATP :
-
SUR1-regulated nonselective cation channel
- NCX:
-
Na+/Ca2+ exchanger
- NF-κB:
-
Nuclear factor-kappaB
- NH4 + :
-
ammonia/ammonium ion
- NHE:
-
Na+/H+ exchanger
- NKCC:
-
Na–K–Cl cotransporter
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- ONS:
-
oxidative/nitrosative stress
- OSR1:
-
oxidative stress response kinase
- PK:
-
protein kinase
- SIN-1:
-
3-morpholinosydnoimine HCl
- SiRNA:
-
Small interfering RNA
- SNAP:
-
S-nitroso-N-acetyl penicillamine
- SPAK:
-
STE20/SPS-1 related, proline-alanine-rich kinase
- TBI:
-
traumatic brain injury
- TBOH:
-
tert-butylhydroperoxide
- WNK1:
-
WNK lysine deficient protein kinase 1
- WNK4:
-
WNK lysine deficient protein kinase 4
References
Abbruscato TJ, Lopez SP, Roder K, Paulson JR (2004) Regulation of blood-brain barrier Na, K, 2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J Pharmacol Exp Ther 310:459–468
Akimova OA, Grygorczyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN (2006) Transient activation and delayed inhibition of Na+, K+, Cl− cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem 281:31317–31325
Andersen GO, Skomedal T, Enger M, Fidjeland A, Brattelid T, Levy FO, Osnes JB (2004) 1-AR-mediated activation of NKCC in rat cardiomyocytes involves ERK-dependent phosphorylation of the cotransporter. Am J Physiol Heart Circ Physiol 286:H1354–H1360
Annunziato L, Pignataro G, Di Renzo GF (2004) Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev 56:633–654
Baldwin AS Jr (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14:649–683
Biros MH, Dimlich RW, Barsan WG (1985) Post-insult treatment of ischemia-induced cerebral lactic acidosis in the rat. Ann Emerg Med 15:397–404
Blei AT, Olafsson S, Therrien G, Butterworth RF (1994) Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology 19:1437–44
Chen H, Sun D (2005) The role of Na–K–Cl co-transporter in cerebral ischemia. Neurol Res 27:280–286
Chen H, Kintner DB, Jones M, Matsuda T, Baba A, Kiedrowski L, Sun D (2007) AMPA- mediated excitotoxicity in oligodendrocytes: role for Na+, K+, Cl− co-transport and reversal of Na+/Ca2+ exchanger. J Neurochem 102:1783–1795
Chin S, Delamere NA (1999) Stimulation of active sodium-potassium transport by hydrogen peroxide in cultured rabbit nonpigmented ciliary epithelium. Curr Eye Res 18:254–260
Di Ciano-Oliveira C, Sirokmány G, Szászi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A (2003) Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol Cell Physiol 285:C555–C566
Diecke FP, Wen Q, Iserovich P, Li J, Kuang K, Fischbarg J (2005) Regulation of Na–K–2Cl cotransport in cultured bovine corneal endothelial cells. Exp Eye Res 80:777–785
DiPolo R, Beaugé L (2006) Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Rev 86:155–203
Dowd BF, Forbush B (2003) PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na–K–Cl cotransporter (NKCC1). J Biol Chem 278:27347–27353
Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA (1996) Localization and regulation of the rat renal Na+, K+, Cl− cotransporter, BSC-1. Am J Physiol 271:F619–F628
Elliott SJ, Schilling WP (1992) Oxidant stress alters Na+ pump and Na+, K+, Cl− cotransporter activities in vascular endothelial cells. Am J Physiol 263:H96–H102
Flatman PW (2008) Cotransporters, WNKs and hypertension: an update. Curr Opin Nephrol Hypertens 17:186–192
Gagnon KB, England R, Delpire E (2006a) Characterization of SPAK and OSR1, regulatory kinases of the Na–K–2Cl cotransporter. Mol Cell Biol 26:689–698
Gagnon KB, England R, Delpire E (2006b) Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290:C134–C142
Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269:17713–17722
Ganz R, Swain M, Traber P, DalCanto M, Butterworth RF, Blei AT (1989) Ammonia-induced swelling of rat cerebral cortical slices: implications for the pathogenesis of brain edema in acute hepatic failure. Metab Brain Dis 4:213–223
Garty H, Benos DJ (1988) Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev 68:309–73
Gimenez I, Forbush B (2003) Short-term stimulation of the renal Na–K–Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278:26946–26951
Haas M (1994) The Na–K–Cl cotransporters. Am J Physiol 267:C869–C885
Haas M, Forbush B III (1998) The Na–K–Cl co-transporters. J Bioenerg Biomembr 30:161–172
Haas M, Forbush B III (2000) The Na–K–Cl co-transporter of secretory epithelia. Ann Rev Physiol 62:515–534
He H, Podymow T, Zimpelmann J, Burns KD (2003) NO inhibits Na+, K+, Cl− cotransport via a cytochrome P-450-dependent pathway in renal epithelial cells (MMDD1). Am J Physiol Renal Physiol 284:F1235–F1244
Hoffmann EK (1985) Role of separate K+ and Cl− channels and of Na+/Cl− cotransport in volume regulation in Ehrlich cells. Fed Proc 44:2513–2519
Hoppe D, Kettenmann H (1989) GABA triggers a Cl− flux from cultured mouse oligodendrocytes. Neurosci Lett 97:334–339
Ibla JC, Khoury J, Kong T, Robinson A, Colgan SP (2006) Transcriptional repression of Na–K–2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol 291:C282–C289
Iwamoto T (2004) Forefront of Na+/Ca2+ exchanger studies: molecular pharmacology of Na+/Ca2+ exchange inhibitors. J Pharmacol Sci 96:27–32
Jayakumar AR, Panickar KS, Murthy ChR, Norenberg MD (2006) Oxidative stress and mitogen- activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci 26:4774–4784
Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B 3rd, Reddy PV, Norenberg MD (2008a) Na–K–Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem 283:33874–33882
Jayakumar AR, Panickar KS, Moriyama M, Liu M, Norenberg MD (2008b) Ion transporting systems in the mechanism of astrocyte swelling after trauma. J Neurochem 104(Suppl 1):135–135
Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD (2008c) Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol 67:417–427
Juncos R, Garvin JL (2005) Superoxide enhances Na–K–2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288:F982–F987
Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D (2009) Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 24:257–265
Kaplan MR, Mount DB, Delpire E (1996) Molecular mechanisms of NaCl cotransport. Annu Rev Physiol 58:649–668
Kimura J, Ono T, Sakamoto K, Ito E, Watanabe S, Maeda S, Shikama Y, Yatabe MS, Matsuoka I (2009) Na+–Ca2+ exchanger expression and its modulation. Biol Pharm Bull 32:325–331
Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D (2007) Role of Na+, K+, Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol 292:C1113–C1122
Klein JD, Perry PB, O’Neill WC (1993) Regulation by cell volume of Na+, K+, Cl− cotransport in vascular endothelial cells: role of protein phosphorylation. J Membr Biol 132:243–252
Kurihara K, Moore-Hoon ML, Saitoh M, Turner RJ (1999) Characterization of a phosphorylation event resulting in upregulation of the salivary Na+, K+, Cl− co-transporter. Am J Physiol 277:C1184–C1193
Lam TI, Anderson SE, Glaser N, O’Donnell ME (2005) Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes 54:510–516
Layne J, Yip S, Crook RB (2001) Down-regulation of Na–K–Cl cotransport by protein kinase C is mediated by protein phosphatase 1 in pigmented ciliary epithelial cells. Exp Eye Res 72:371–379
Lenart B, Kintner DB, Shull GE, Sun D (2004) Na–K–Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci 24:9585–9597
Liang D, Bhatta S, Gerzanich V, Simard JM (2007) Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg Focus 22:E2
Lu KT, Wu CY, Cheng NC, Wo YY, Yang JT, Yen HH, Yang YL (2006) Inhibition of the Na+, K+, Cl− -cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur J Pharmacol 548:99–105
Lytle C, Forbush B 3rd (1992) The Na–K–Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem 267:25438–25443
MacMillan-Crow LA, Greendorfer JS, Vickers SM, Thompson JA (2000) Tyrosine nitration of c-SRC tyrosine kinase in human pancreatic ductal adenocarcinoma. Arch Biochem Biophys 377:350–356
Mallozzi C, Di Stasi AM, Minetti M (2001) Nitrotyrosine mimics phosphotyrosine binding to the SH2 domain of the src family tyrosine kinase lyn. FEBS Lett 503:189–195
Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA (1998) Na–K–2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl- secretory capacity by butyrate. J Clin Invest 101:2072–2079
Meima ME, Mackley JR, Barber DL (2007) Beyond ion translocation: structural functions of the sodium-hydrogen exchanger isoform-1. Curr Opin Nephrol Hypertens 16:365–372
Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H (2005) WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280:42685–42693
Murthy CR, Rama Rao KV, Bai G, Norenberg MD (2001) Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res 66:282–288
Nagaraja TN, Brookes N (1998) Intracellular acidification induced by passive and active transport of ammonium ions in astrocytes. Am J Physiol 274:C883–C891
Norenberg MD (1977) A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest 36:618–627
Norenberg MD (1987) The role of astrocytes in hepatic encephalopathy. Neurochem Pathol 6:13–33
Norenberg MD, Bender AS (1994) Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien) 60:24–27
Norenberg MD, Rama Rao KV, Jayakumar AR (2009) Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis 24:103–117
O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE (2006) Estradiol reduces activity of the blood-brain barrier Na–K–Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26:1234–1249
O’Mahony F, Toumi F, Mroz MS, Ferguson G, Keely SJ (2008) Induction of Na+, K+, Cl− cotransporter expression mediates chronic potentiation of intestinal epithelial Cl- secretion by EGF. Am J Physiol Cell Physiol 294:C1362–C1370
Ortiz PA, Hong NJ, Garvin JL (2001) NO decreases thick ascending limb chloride absorption by reducing Na+, K+, Cl− cotransporter activity. Am J Physiol Renal Physiol 281:F819–F825
Paljärvi L (1984) Brain lactic acidosis and ischemic cell damage: a topographic study with high- resolution light microscopy of early recovery in a rat model of severe incomplete ischemia. Acta Neuropathol 64:89–98
Payne JA, Forbush B 3rd (1994) Alternatively spliced isoforms of the putative renal Na–K–Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci U S A 91:4544–4548
Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U, Abbruscato TJ (2009) Nicotine Exacerbates Brain Edema during in vitro and in vivo Focal Ischemic Conditions. J Pharmacol Exp Ther (in press)
Pewitt EB, Hegde RS, Haas M, Palfrey HC (1990) The regulation of Na/K/2Cl cotransport and bumetanide binding in avian erythrocytes by protein phosphorylation and dephosphorylation. Effects of kinase inhibitors and okadaic acid. J Biol Chem 265:20747–20756
Piechotta K, Garbarini N, England R, Delpire E (2003) Characterization of the interaction of the stress kinase SPAK with the Na+, K+, Cl- cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem 278:52848–52856
Plato CF, Stoos BA, Wang D, Garvin JL (1999) Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol 276:F159–F163
Pond BB, Berglund K, Kuner T, Feng G, Augustine GJ, Schwartz-Bloom RD (2006) The chloride transporter Na+, K+, Cl− cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J Neurosci 26:1396–1406
Rama Rao KV, Jayakumar AR, Norenberg DM (2003) Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab Brain Dis 18:113–127
Ringel F, Chang RC, Staub F, Baethmann A, Plesnila N (2000) Contribution of anion transporters to the acidosis-induced swelling and intracellular acidification of glial cells. J Neurochem 75:125–132
Rose CR, Waxman SG, Ransom BR (1998) Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci 18:3554–3562
Russell JM (2000) Sodium-potassium-chloride co-transport. Physiol Rev 80:211–276
Salter MG, Fern R (2008) The mechanisms of acute ischemic injury in the cell processes of developing white matter astrocytes. J Cereb Blood Flow Metab 28:588–601
Selvaraj NG, Omi E, Gibori G, Rao MC (2000) Janus kinase 2 (JAK2) regulates prolactin- mediated chloride transport in mouse mammary epithelial cells through tyrosine phosphorylation of Na+, K+, Cl− cotransporter. Mol Endocrinol 14:2054–2065
Shumaker H, Soleimani M (1999) CFTR upregulates the expression of the basolateral Na+, K+, Cl− cotransporter in cultured pancreatic duct cells. Am J Physiol 277:C1100–C1110
Siesjo BK (1981) Cell damage in the brain: a speculative synthesis. J Cerebr Blood Flow Metab 1:155–185
Silver IA, Deas J, Erecińska M (1997) Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience 78:589–601
Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, Reddy PVB, Norenberg MD (2008) NF-κB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem 106:2302–2311
Staub F, Stoffel M, Berger S, Eriskat J, Baethmann A (1994) Treatment of vasogenic brain edema with the novel Cl− transport inhibitor torasemide. J Neurotrauma 11:679–690
Stys PK, Waxman SG, Ransom BR (1992) Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na+–Ca2+ exchanger. J Neurosci 12:430–439
Su G, Kintner DB, Sun D (2002) Contribution of Na+, K+, Cl− cotransporter to high [K+]o− induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol 282(5):C1136–C1146
Sun D, O’Donnell ME (1996) Astroglial-mediated phosphorylation of the Na–K–Cl cotransporter in brain microvessel endothelial cells. Am J Physiol 271:C620–C627
Swain MS, Blei AT, Butterworth RF, Kraig RP (1991) Intracellular pH rises and astrocytes swell after portacaval anastomosis in rats. Am J Physiol 261:R1491–R1496
Takahashi H, Koehler RC, Brusilow SW, Traystman RJ (1991) Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemia in rats. Am J Physiol 281:H826–H829
Thomas R, Salter MG, Wilke S, Husen A, Allcock N, Nivison M, Nnoli AN, Fern R (2004) Acute ischemic injury of astrocytes is mediated by Na–K–Cl cotransport and not Ca2+ influx at a key point in white matter development. J Neuropathol Exp Neurol 63:856–871
Traber PG, Dal Canto M, Ganger DR, Blei AT (1987) Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272–1277
Unterberg AW, Stover J, Kress B, Kiening KL (2004) Edema and brain trauma. Neuroscience 129:1021–1029
Vitari AC, Deak M, Morrice NA, Alessi DR (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391:17–24
Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397:223–231
Wang H, Yan Y, Kintner DB, Lytle C, Sun D (2003) GABA-mediated trophic effect n oligodendrocytes requires Na–K–2Cl co-transport activity. J Neurohysiol 90:1257–1265
Wong JA, Gosmanov AR, Schneider EG, Thomason DB (2001) Insulin-independent, MAPK- dependent stimulation of NKCC activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 281:R561–R571
Worrell RT, Merk L, Matthews JB (2008) Ammonium transport in the colonic crypt cell line, T84: role for Rhesus glycoproteins and NKCC1. Am J Physiol Gastrointest Liver Physiol 294:G429–G440
Xie Y, Dengler K, Zacharias E, Wilffert B, Tegtmeier F (1994) Effects of the sodium channel blocker tetrodotoxin (TTX) on cellular ion homeostasis in rat brain subjected to complete ischemia. Brain Res 652:216–224
Xu JC, Lytle C, Zhu TT, Payne JA, Benz E Jr, Forbush B 3rd (1994) Molecular cloning and functional expression of the bumetanide-sensitive Na–K–Cl cotransporter. Proc Natl Acad Sci U S A 91:2201–2205
Yan YP, Dempsey RJ, Sun D (2001) Na+, K+, Cl− co-transporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab 21:711–721
Zima AV, Blatter LA (2006) Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71:310–321
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs and by National Institutes of Health Grant DK063311. A.R.J is supported by the American Association for the Study of Liver Disease/American Liver Foundation Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, A.R., Norenberg, M.D. The Na–K–Cl Co-transporter in astrocyte swelling. Metab Brain Dis 25, 31–38 (2010). https://doi.org/10.1007/s11011-010-9180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-010-9180-3