Abstract
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) is well recognized as a critical regulator of redox, metabolic, and protein homeostasis, as well as the regulation of inflammation. An age-associated decline in NRF2 activity may allow oxidative stress to remain unmitigated and affect key features associated with the aging phenotype, including telomere shortening. Telomeres, the protective caps of eukaryotic chromosomes, are highly susceptible to oxidative DNA damage, which can accelerate telomere shortening and, consequently, lead to premature senescence and genomic instability. In this review, we explore how the dysregulation of NRF2, coupled with an increase in oxidative stress, might be a major determinant of telomere shortening and age-related diseases. We discuss the relevance of the connection between NRF2 deficiency in aging and telomere attrition, emphasizing the importance of studying this functional link to enhance our understanding of aging pathologies. Finally, we present a number of compounds that possess the ability to restore NRF2 function, maintain a proper redox balance, and preserve telomere length during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
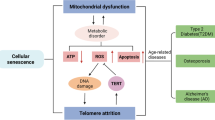
Aging is a biological process that is characterized by a gradual decline in cellular and physiological functions. Many factors contribute to aging, including the accumulation of macromolecular damage in response to a variety of environmental and endogenous stressors. This accumulation of the damage over time and across tissues is a primary risk factor in the genesis of many age-related diseases, such as cardiovascular disease (CVD), cancer, neurodegeneration, diabetes, osteoporosis, and sarcopenia [1, 2]. There is currently evidence that the rate of biological aging is driven by an interconnected network of molecular biological processes that contributes to the development of age-related conditions. However, many of these processes, such as genomic instability, mitochondrial dysfunction, and telomere shortening, are associated with chronic oxidative stress caused by elevated levels of reactive oxygen species (ROS) [3, 4]. Oxidative stress occurs when the cellular balance between the production of ROS and antioxidant defenses is disrupted. This altered redox status is accompanied by deleterious effects on cell survival, including oxidative modifications on biological macromolecules. Although there is a growing consensus that ROS act as mediators in a variety of signaling pathways, during aging, accumulation of defective/damaged organelles, especially mitochondria, leads to uncontrolled levels of ROS production. Likewise, prolonged exposure to xenobiotics or excessive production of pro-inflammatory cytokines may increase the levels of intracellular ROS and contribute to cell injury through oxidative damage [5, 6]. Under conditions of oxidative stress, ROS causes various types of genotoxic stress, including dual damage at telomeres and mitochondria [7].
All aerobic organisms have evolved critical defense mechanisms to combat the harmful effects of intrinsic and extrinsic oxidative insults and preserve homeostatic functions. Although numerous endogenous antioxidant systems are in place to scavenge and maintain proper ROS levels, the activation of nuclear factor erythroid 2-related factor 2 (NFE2L2; more commonly known as NRF2) plays a pivotal role in the maintenance of the cellular redox homeostasis. NRF2 is a basic leucine zipper (bZIP) transcription factor with a cap ‘n’ collar (CNC) structure. It is ubiquitously expressed and present in various organs, including the kidney, muscle, lung, brain, liver, and heart [8]. Under basal conditions, NRF2 is tightly regulated by Kelch-like ECH-associated protein 1 (KEAP1), a repressor protein that mediates the degradation of NRF2 by the ubiquitin–proteasome pathway. Once activated by oxidative stress, NRF2 translocates to the nucleus where it binds to antioxidant response elements (ARE) in the promoter region of genes involved in redox regulation, xenobiotic metabolism, DNA repair, apoptosis, proteostasis, and regulation of inflammation [9]. In addition to its roles in defending cells against electrophilic insults and oxidative damage, NRF2 may be a modulator of longevity-related pathways associated with longer lifespan and healthspan in long-lived species and humans with exceptional longevity [10,11,12]. However, mounting evidence suggests a gradual reduction of NRF2 with age, leading to unmitigated oxidative stress and contributing to the aging phenotype. Furthermore, an age-associated loss of NRF2 function may play a role on many of the cellular and molecular processes known to drive aging (i.e., hallmarks of aging), including cellular senescence, mitochondrial dysfunction, and telomere attrition [13, 14].
Among the many molecular changes associated with old age, telomere length (TL) has been recognized as one of the best biomarkers of aging. Despite pre-analytical and analytical issues, hampering the implementation of TL as routine marker into clinical practice, human studies have found a significant inverse relationship between TL and several age-related conditions [15, 16]. Telomeres are the genomic portions at the ends of linear chromosomes. They are specialized structures that maintain the structural integrity of chromosomes. However, at each somatic cell division cycle, human telomeres lose 50–200 base pairs due to incomplete synthesis of the lagging strand during the DNA replication [17]. In addition, data from several studies indicate that telomeric DNA is hypersensitive to oxidative damage, a phenomenon recently named TelOxidation [18]. Accordingly, enhanced levels of oxidative stress may cause an accelerated telomere shortening, increasing the rate of developing age-related diseases [19]. It has been proposed that oxidative stress and free radicals play a crucial role in telomere shortening by altering both telomerase activity and telomere repeat binding factor 2 (TRF-2) expression levels [20].
As NRF2 plays a crucial role in protecting cells from oxidative damage, an age-related decline in NRF2 function could be a key driving force behind telomere attrition. Here, we provide an overview of the molecular basis regulating the functional link between NRF2 and telomeres in aging and age-related diseases, also highlighting novel strategies to preserve their role in cellular homeostasis.
Role of NRF2 in oxidative stress
All eukaryotic organisms have evolved sophisticated mechanisms to manage oxidative and electrophilic insults that arise from internal metabolic reactions or xenobiotic challenges. NRF2 has evolved over millennia to become a versatile transcription factor that induces a broad range of biological responses, including detoxification and antioxidant activities. Recent advances in our understanding of the NRF2 pathway have revealed that this system is related to a number of age-related diseases, such as cancer, neurodegenerative diseases, and diabetes mellitus [21, 22].
The NRF2 signaling pathway can regulate more than 600 genes and some of these genes encode cytoprotective proteins that are also associated with regulation of inflammatory response [9]. In humans, NRF2 is composed of 605 amino acids and 7 highly conserved NRF2-ECH homology domains (Neh 1–7) (Fig. 1). The Neh1 domain binds to the ARE sequence using a bZIP motif [23]. In this region, NRF2 will heterodimerize with small musculoaponeurotic fibrosarcoma proteins (sMAF) to induce the transcription of NRF2 target genes. The Neh2 domain interacts with Keap1 using the DLG (Asp-Leu-Gly) and ETGE (Glu-Thr-Gly-Glu) motifs, while Neh 3, 4, and 5 domains are required to promote the transactivation of several cytoprotective genes. The Neh6 domain controls the degradation of NRF2 by binding β-transducin repeat-containing protein (β-TrCP) and with a KEAP1-independent mechanism. The Neh7 domain interacts with the retinoic acid receptor α (RARα), a nuclear receptor that is known to suppress the activity of NRF2 pathway and decrease ARE gene expression [24].
Functional domains of human NRF2. NRF2 has seven highly conserved domains (Neh1–Neh7). Neh1 contains a CNC basic-region leucine zipper domain that interacts with sMAF and DNA. Neh2 contains two recognition motifs (ETGE and DLG), which are required for binding with KEAP1. Neh3, Neh4, and Neh5 domains are necessary for transactivation. Neh6 is involved in a KEAP1-independent degradation of NRF2 by binding β-TrCP. Neh7 interacts with RARα suppressing the activity of NRF2. ARE antioxidant response elements, β-TrCP binding β-transducin repeat-containing protein, CNC cap ‘n’ collar, KEAP1 kelch-like ECH-associated protein 1, Neh NRF2-ECH homology domains, RARα retinoic acid receptor α, sMAF small MAF proteins
Although the activation of NRF2 may be regulated by multiple mechanisms, the canonical pathway involves a KEAP1-dependent activation of NRF2 [25]. Indeed, as mentioned above, before oxidative/electrophilic stress, NRF2 interacts in the cytosol with its repressor KEAP1, a thiol-rich protein containing highly reactive cysteines. However, KEAP1 is not only a factor involved in regulating NRF2 inhibition and turnover, but it may also be a sensor of oxidative stimuli. Under homeostatic conditions, KEAP1 can interact with the Cullin 3 (Cul3)-based ubiquitin ligase E3 complex that leads to the proteasomal degradation of NRF2. Conversely, increased oxidative/electrophilic stress can induce modifications in specific cysteine residues, causing a conformational change in the NRF2-KEAP1 complex that prevents the ubiquitylation of de novo NRF2 [26].
The cis-acting enhancer ARE sequence is found in the promoter region of multiple genes regulated by NRF2, including various genes encoding antioxidant enzymes involved in restoring a stable internal environment after an oxidative insult. For example, NRF2 promotes the generation of nicotinamide adenine dinucleotide phosphate (NADPH), a critical cofactor that aids antioxidant reactions, by regulating the expression of genes such as glucose 6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (6PGD), malic enzyme 1 (ME1), and isocitrate dehydrogenase 1 (IDH1) [27]. NADPH is further used as a reducing equivalent by a large number of redox reactions, many of which are regulated by NRF2 [28]. A key mechanism involved in the restoration of redox homeostasis is the glutathione antioxidant pathway, present primarily in the reduced form [reduced glutathione (GSH)]. NRF2 controls the levels of critical enzymes involved in the synthesis of GSH, such as the catalytic and modulator subunits of glutamate–cysteine ligase (GCLC, GCLM), glutathione reductase (GR), glutathione peroxidase (GPX), and several glutathione S-transferases (GST) [29]. A number of studies have shown that an age-related decline in NRF2 activity can decrease the intracellular GSH pool due to reduced expression of GCLC and GCLM [30, 31]. Other important redox genes regulated by NRF2 include superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), peroxiredoxin (PRDX), sulfiredoxin1 (SRXN1), and the thioredoxin (TRX)-based antioxidant system (Fig. 2 includes other important targets of NRF2). These enzymes participate in metabolic reactions that scavenge ROS and neutralize electrophiles, but they can undergo a gradual decline with age [32, 33]. Therefore, the impairment of NRF2 function during aging may be a key feature of a wide variety of age-related diseases (Fig. 3) characterized by elevated levels of reactive species. Moreover, accumulating data indicate that the decline of NRF2 associated with aging may exacerbate the progression of these pathologies, leading to the increased expression of pro-inflammatory biomarkers. Nuclear factor kappa B (NF-κB) is a redox-regulated transcription factor that controls the activation of many pro-inflammatory genes [34]. ROS-induced damage accumulation caused by an attenuated activity of NRF2 during aging may induce NF-κB and increase the expression pro-inflammatory mediators, such as interleukin-1-beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and many others [35]. It has been shown that there is a functional relationship between NRF2 and NF-κB to maintain redox homeostasis and regulate the cellular response to stress and inflammation [36]. Depletion of NRF2 may induce more aggressive inflammation through activation of NF-κB and downstream pro-inflammatory cytokines [37]. Conversely, the activation of the NRF2 pathway is often associated with a decreased activity of NF-κB resulting in attenuation of inflammatory reactions. A large number of enzymes controlled by NRF2, such as HO1, GCLC, and NQO1, may inhibit both cytokines and chemokines, including TNF-α, IL-6, IL-1β, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-2 (MIP2) [38]. Therefore, it is clear that the crosstalk between NRF2 and NF-κB plays an important role in regulating inflammation and oxidative defense system.
Repression and activation of NRF2 transcriptional network. NRF2 binds to KEAP1 under basal conditions, resulting in its rapid proteasomal degradation. The canonical mechanism for NRF2 activation relies on conformational changes in the KEAP1 structure induced by stress stimuli, which prevents the KEAP1–NRF2 interaction. NRF2 accumulates in the nucleus and facilitates transcription or repression of its target genes by binding to AREs. AKR aldo–keto reductase, AOX1 aldehyde oxidase, ARE antioxidant response elements, ARG-1 arginase 1, CAT catalase, CES carboxylesterases, Cul3 Cullin 3, FIZZ-1 found in inflammatory zone 1, FTH ferritin heavy chain, FTL ferritin light chain, G6PDH glucose-6-phosphate dehydrogenase, GCL γ-glutamylcysteine synthetase, GPX glutathione peroxidase, GSR glutathione reductases, GST glutathione S-transferase, HO1 heme oxygenase-1, IDH1 isocitrate dehydrogenase, IFN interferon, IL-1β interleukin 1β, IL-4 interleukin 4, IL-6 interleukin 6, IL-10 interleukin 10, KEAP1 kelch-like ECH-associated protein 1, ME1 malic enzyme 1, MRP multidrug resistant protein, MT metallothionein, MTHFD2 methylenetetrahydrofolate dehydrogenase, NQO1 NADPH quinone oxidoreductase-1, OATP2 organic anion transporting polypeptide 2, PGD phosphogluconate dehydrogenase, PPAT phosphoribosyl pyrophosphate amidotransferase, PRDX peroxiredoxin, SLC7A11 solute carrier family 7 member 11, sMAF small MAF proteins, SOD1 Cu–Zn superoxide dismutase, SRXN sulfiredoxin, TNFα tumor necrosis factor α, TRXR thioredoxin reductase, TRX thioredoxin, UGT UDP-glucuronosyltransferase
NRF2 in aging and age-related diseases
As mentioned above, the age-dependent impairment of antioxidant enzymes has been linked to a decline in the transcriptional activity of NRF2. Defective antioxidant defense mechanisms promote a pro-oxidant environment driving, at least partially, the aging phenotype and contributing to the progression of age-related diseases [39]. The imbalance between oxidants and antioxidant enzymes caused by an age-related reduction of NRF2 activity may lead to defective autophagic activities. Indeed, it has been reported that NRF2 activates both chaperone-mediated autophagy and macroautophagy, participating in the clearance of oxidized or damaged proteins during redox alterations [40, 41]. Proteotoxicity, one of the hallmarks of aging, is characterized by the accumulation of toxic misfolded proteins. An age-related decline in NRF2 function impairs the unfolded protein response (UPR), a signal transduction pathway that prevents the accumulation of misfolded proteins [42].
Aging is also characterized by a gradual decline of DNA damage repair mechanisms, resulting in an increased frequency of mutations. DNA repair proteins, such as RAD51 homolog 1 (RAD51) and p53-binding protein (53BP1), are target genes of NRF2. RAD51 and 53BP1 may facilitate the repair of DNA in both homologous recombination (HR) and non-homologous end-joining (NHEJ) [43, 44]. Therefore, reduced activity of NRF2 during aging may increase genomic instability and inhibit DNA repair mechanisms. The effect of NRF2 loss in aging could also play a role in mitochondrial dysfunction and cellular senescence. Firstly, NRF2 maintains mitochondrial function by controlling fatty acid oxidation and modulating the mitochondrial permeability transition pores (MPTP) [45]. Secondly, NRF2 can reduce the senescence-associated secretory phenotype (SASP) by modulating oxidative stress, endoplasmic reticulum stress, inflammation, immune response, and cell cycle arrest [46]. An age-related decline in NRF2 activity may also contribute to the deregulation of nutrient-sensing pathways, such as the AMP-activated protein kinase (AMPK), that are associated with aging and age-related pathogenesis [47].
Given that NRF2 controls a wide range of biological processes, including antioxidant enzyme response, a decline in its efficiency during aging may lead to the accumulation of damaged cellular components. Accordingly, unmitigated and elevated levels of ROS can increase susceptibility to neurodegeneration, cancer, and other age-related pathologies. Elevated levels of oxidation products, such as protein nitrotyrosine, carbonyls in proteins, lipid oxidation products, and oxidized DNA bases, have been observed in postmortem brains from Alzheimer’s disease (AD) patients, suggesting an increase in the oxidation of macromolecules in these patients [48]. It is also found that NRF2 nuclear localization is diminished in AD neurons and many ARE-containing genes are downregulated. This deficiency is also accompanied by an elevation of pro-inflammatory molecules, such as TNF-α, IL-1β, and IL-6 [49, 50]. Moreover, NRF2 reduces the levels of hyperphosphorylated tau, a neuronal feature of AD pathology, by enhancing its autophagic degradation [51]. The functional decline of NRF2 and its target genes with aging appears to be a major risk factor in the progression of Parkinson’s disease (PD) [52]. NRF2 may protect dopaminergic neurons by maintaining mitochondrial quality control in neuronal cells. In PD, it has been demonstrated that many antioxidant enzymes regulated by NRF2 are sequestered in Lewy bodies and, therefore, they are unable to reduce oxidative stress associated with the loss of dopaminergic neurons in the substantia nigra [53].
It is now well established that many types of human cancer are age-related diseases. Accumulating evidence suggests that NRF2 has a contradictory role in cancers; however, it plays a key role in DNA repair mechanisms by reducing genomic instability occurring in cancer cells [54]. Accordingly, the decline of NRF2 with aging may lead to tumorigenesis by increasing DNA damage and mutations. Moreover, NRF2 target genes are also critical mediators to enhance the metabolism of pro-tumorigenic xenobiotics. A transcriptional decrease of xenobiotic-metabolizing genes may be a key driver of cancer initiation in an aging population. At the same time, NRF2 and its downstream genes are overexpressed in many human cancer tissues, providing a survival and growth advantage to cancer cells. Although NRF2 activation continues to represent a crucial strategy for preventing or slowing tumorigenesis, the temporal and contextual nature of NRF2 modulation in cancer remains a complicated balancing act. [55, 56].
Sarcopenia, a progressive disorder characterized by a significant loss of muscle mass and muscle function, is a major health condition associated with aging [57]. There is also a secondary sarcopenia caused by diseases such as cancer, chronic obstructive pulmonary disease (COPD), heart failure, renal failure, and many others [58]. A relationship between NRF2 deficiency and muscle loss has been observed during aging. Many studies have demonstrated that a decrease in NRF2 activity causes muscle loss by impairing antioxidant mechanisms and muscle regeneration. In addition, NRF2 deficiency may exacerbate sarcopenia during aging by impairing skeletal muscle mitochondrial biogenesis and dynamics [59, 60]. There is also evidence that age-related deregulation of NRF2 is implicated in the pathophysiology of various conditions, including CVD, diabetes, osteoporosis, and ocular disease [22, 61].
Impact of oxidative damage on telomere shortening
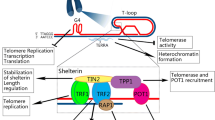
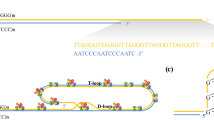
Telomeres are specialized structures formed by telomeric DNA and binding proteins. In mammals, they are highly conserved tandem repeats of the sequence TTAGGG. Telomeres are located at the end of each chromosome arm to preserve chromosomal structural integrity and maintain genomic stability. The repetitive TTAGGG sequences are bound by a group of protective proteins known as the “shelterin” complex. A detailed description of the shelterin proteins is given in Table 1. Shelterin maintains genome integrity by capping the chromosome terminus, it is implicated in the generation of T-loops, and it controls the DNA damage response (DDR) [62, 63]. Progressive telomere shortening occurs during cell division due to the inability of the DNA polymerase to synthesize in a 3′–5′ direction, leading to the incomplete replication of the lagging strand. Telomere shortening is a major driver of cellular senescence and cellular growth arrest. These phenomena exert a tumor-suppressive effect in long-lived animals, including humans. However, telomere shortening can also lead to genomic instability and promote cancer progression [64]. Telomerase, a ribonucleoprotein (RNP) complex composed of the telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC), overcomes the end-replication problem of eukaryotic chromosomes. The long non-coding RNA (lncRNA) is an essential constituent of TERC containing template for telomeric DNA synthesis in association with TERT which serves as the catalytic component [65]. A second lncRNA is the telomeric repeat-containing RNA (TERRA) which is transcribed from the subtelomeric region and helps in regulating TL [66].
The semiconservative nature of DNA replication implies that telomere loss may occur in a stochastic fashion [67]. Moreover, there is a large body of literature suggesting that telomere shortening is influenced by numerous factors other than the end replication problem or difficulties associated with the DNA replication machinery [68, 69]. The highly repetitive G-rich telomeric repeats are preferred sites for production of 8-oxoguanine (8-oxoG); therefore, telomeric DNA is remarkably susceptible to oxidative stress. When telomeres reach a critically short threshold, cells undergo telomere-induced (or replicative) senescence. The formation of the adduct 8-oxoguanine (8-oxoG) in the telomeric region, one of the predominant forms of free radical-induced oxidative lesions, may lead to the production of transversions (i.e., replacement of a purine with a pyrimidine or vice versa) and promote different types of cancer. Recent data revealed that persistent accumulation of 8-oxoG at telomeres promotes telomere loss and provides a mechanism by which this common oxidative lesion may drive overall genomic instability [70]. Indeed, chronic formation and persistence of 8-oxoG at telomeres not only promote telomere shortening but also chromosome end fusions and chromatin bridges. 8-oxoG can promote end-to-end fusions by causing telomere loss and crisis, which can occur due to the false recognition of “uncapped” chromosome ends as double-strand breaks (DSBs) by DDR signaling and end-joining pathways [71]. After exposure to ROS and accumulation of 8-oxoG lesions, telomeric DNA exhibits a high frequency of single-strand break formation in the DNA backbone [72]. Likewise, oxidative base damage or base loss can also block polymerases leading to telomeric double-strand breaks. 8-oxoG in duplex DNA is primarily repaired by base excision repair (BER) but the activity of BER decreases with age in multiple tissues, contributing to age-related occurrence of cancer and other age-related pathologies [73]. Several lines of evidence also indicate that the repair of oxidative lesions is less efficient in telomeres than in the rest of the genome [74, 75]. This repair deficiency in telomeres may be due to different reasons, including the overexpression of TERF-2, a component of the shelterin complex involved in the protection of chromosome ends by promoting the formation of the T-loop structure. This telomeric protein blocks the access of DNA repair enzymes to telomeric strand breaks and inhibits the phosphorylation of enzymes involved in the DDR [76, 77]. It has also been reported that oxidized telomeric DNA, particularly a telomeric region containing 8-oxoG, cannot be extended by telomerase. In addition, 8-oxoG disrupts or alters G-quadruplex structures which are inhibitory to telomerase [78, 79].
Although a variety of NRF2-dependent repair proteins are involved in processing oxidative base damage, the antioxidant enzyme PRDX1 may be recruited to telomeres during the S phase of the cell cycle, reducing the abundance of ROS near telomeres and preserving telomeric DNA for extension by telomerase [72]. However, other cellular antioxidant defense systems, such as the glutathione system, CAT, and SOD, diminish ROS and therefore may contribute to telomere protection (Fig. 4) [80, 81].
NRF2-mediated protection of telomeres from oxidative damage. NRF2-mediated transcription of PRDX1 and other cellular antioxidant defense systems diminish oxidative damage contributing to telomere protection. 8-oxoG 8-oxoguanine, ARE antioxidant response elements, CAT catalase, GPX glutathione peroxidase, GST glutathione S-transferase, GTP guanosine-5′-triphosphate, PRDX peroxiredoxin, sMAF small MAF proteins, SOD Cu–Zn superoxide dismutase
Another important point is that the rate of telomere shortening may be further increased by inflammation. Chronic low-grade inflammation might enhance telomere attrition by increasing ROS-mediated DNA damage and accelerate the accumulation of senescent cells [68]. Senescent cells secrete a complex set of pro-inflammatory mediators, known as the senescence-associated secretory phenotype (SASP). SASP acts in a paracrine fashion and induces senescence in surrounding cells, thereby causing systemic chronic inflammation [82]. Numerous human studies have shown that the cumulative load of high inflammation markers, such as IL-6, TNF-α, ICAM-1, MCP-1, and C-reactive protein (CRP), is accompanied by increased risk for short TL or telomere dysfunction. The correlation between inflammatory molecules and shorter telomeres is thought to contribute to the aging process and related diseases [83,84,85,86].
Telomere length and age-related diseases
Although telomere shortening is a well-known hallmark of aging, the clinical value of TL in age-related pathologies and mortality is a hotly debated topic. However, TL remains an informative marker to assess biological age especially when used along with other indices associated with epigenetic alterations, homeostatic dysregulation, and frailty [16]. Epidemiologic studies have repeatedly demonstrated that individuals with shorter telomeres had a mortality rate nearly twice that of those with longer telomeres [87]. In addition, an accelerated rate of telomere shortening may help to predict the risk of future age-related pathologies, such as neurodegenerative diseases, cancer, CVD, and osteoporosis [87,88,89,90,91].
In highly proliferative tissues (e.g., skin and hematopoietic system), continuous tissue renewal and low levels of telomerase in progenitor cell compartments may cause progressive telomere attrition over the years. Likewise, ROS-induced damage of telomere sequences in slowly proliferative tissues (e.g., brain, heart, and liver) accelerates attrition and uncapping over time [92]. Moreover, senescence-associated inflammation has been connected to shorter telomeres and is thought to drive multiple age-related diseases [19]. The relationship between telomere shortening, inflammation, and senescence has recently emerged as an important player in neurodegenerative diseases. It has been shown that TERT may play a significant role in T cell senescence and neurodegenerative diseases by regulating oxidative stress and inflammatory responses and slowing down telomere attrition. Neurons of TERT knockout mice showed shorter telomeres, increased production of oxidative species, and an increase in cellular oxidative damage in response to pathological tau [93, 94]. Despite this, TL is not only associated with AD risk but also with disease progression. A study from Tedone et al. indicates that late-onset AD patients with slow disease progression had shorter telomeres than patients with fast disease progression or healthy elderly controls [95]. It has also been shown that telomere shortening in T cells correlates with AD disease status, measured by mini mental status exam (MMSE) [96].
The role of shortened TL in cancer is highly complex and the relationship between oxidative damage and short telomeres in the development and perpetuation of cancer is subject to intensive research. Oxidative stress may contribute to the genome instability of cancerous cells and there is evidence that the telomeres from cancer cells (e.g., breast, colon, and prostate) are shorter than in healthy tissue from the same organ [97,98,99]. A number of studies found an association between increased incidence of cancer and accelerated telomere shortening. For example, the Normative Aging Study found that individuals who developed cancer during the follow-up experienced a telomere shortening rate that was twice as high as those without cancer [100]. The link between shortened telomeres and cancer risk has been subsequently validated in several meta-analyses. Specifically, compelling evidence has been gathered regarding the elevated risk of gastrointestinal, head, and neck cancers associated with shortened telomeres [101]. Despite the tendency of most studies to ignore the variation in TL across chromosomes and cells, it appears that the variability of TL may serve as a risk factor for cancer-related mortality. However, some studies did not observe significant connections between TL and cancer risk. Conversely, certain studies have reported higher TL in cancer patients compared to individuals without cancer [102, 103]. A Mendelian randomization study showed that genetically increased TL is associated with increased risk of several cancers. The protection of telomeres 1 (POT1) protein is an essential subunit of the shelterin telomere binding complex and appears to be most commonly involved in cancer. Recent clinical findings suggest POT1 mutations associated with long TL confer a predisposition to a range of benign and malignant solid neoplasms [104, 105].
Multiple prospective cohort studies have indicated that individuals with a shorter TL and a faster rate of telomere attrition are at a heightened risk of CVD, myocardial infarction, heart failure, and stroke [106,107,108,109]. Furthermore, Carty et al. found that leukocyte TL (LTL) has the potential to predict mortality in CVD patients [110]. It has also been shown that patients with acute coronary syndrome exhibit shortened telomeres, which were correlated with the presence of highly unstable atherosclerotic plaques. Additionally, these individuals also had elevated levels of oxidative stress and inflammatory mediators [111]. Various studies have indicated that diabetes mellitus, a common risk factor for CVD, is associated with shorter telomeres. Individuals who have both short telomeres and diabetes are characterized by a faster disease progression and an increased susceptibility to various diabetic complications, such as retinopathy, nephropathy, neuropathy, and peripheral vascular disease [112, 113]. Similar to numerous other tissues, bone cells also exhibit an age-related decrease in TL. Telomere shortening may play a role in the onset and progression of osteoporosis, which is a prevalent condition among elderly individuals. Despite some conflicting results, a large population-based study in 2150 women provided evidence that shortened LTL is associated with a decrease in bone mineral density and the presence of osteoporosis [88, 114].
Influence of NRF2 on telomere length
An intriguing interplay between NRF2 and TL regulation is increasingly supported by emerging studies (Table 2). There is evidence that human immunodeficiency virus (HIV) infection is a model of accelerated aging associated with the premature development of age-related comorbidities, such as CVD, neurodegeneration, and metabolic syndrome. HIV is also accompanied by a disruption of the redox balance and a decrease in the protein function of NRF2, which promotes the acquisition of a premature senescence phenotype [115]. It has been found that antiretroviral therapy, a recognized risk factor for CVD, decreases TL by activating the serine/threonine kinase p90RSK and inhibiting NRF2-ARE activity. The involvement of p90RSK-NRF2 signaling in HIV accelerates the aging process by regulating four components of the senescent phenotype: (1) DNA damage caused by telomere shortening, leading to the induction of p16 and p21, (2) generation of ROS, (3) inflammation, and (4) impairment of efferocytosis induced by antiretroviral therapy [116].
The connection between NRF2 and telomeres has been revealed in the context of antitelomerase therapy, which holds promise for the treatment of cancer. This therapy may provoke alternative lengthening of telomeres (ALT), an alternative mechanism for telomere maintenance. It has been reported that NRF2 may be implicated in the adaptive response of cancer cells undergoing antitelomerase therapy, potentially influencing ALT activation and mitochondrial adaptive mechanisms [117]. An association between NRF2 and TERT has been demonstrated in many studies. Gong et al. reported that human TERT interacts with the transcription factor Y-box binding protein 1 (YBX1), promoting the formation of a complex that binds to the NRF2 promoter region. This interaction leads to increased NRF2 expression and contributes to cell proliferation [118]. It has also been found that NRF2 activates the transcription of X-ray repair cross-complementing 5 (XRCC5), a DNA repair gene, which subsequently enhances the expression of human TERT. This upregulation of XRCC5 and human TERT by NRF2 contributes to enhanced DNA repair mechanisms and telomere maintenance [119]. NRF2 may also attenuate ferroptosis, a form of regulated cell death, promoting the expression of telomere-associated genes, including TERT and solute carrier family 7 member 11 (SLC7A11). The increase of SLC7A11 by NRF2 contributes to telomere maintenance and enhances cellular antioxidant defenses, reducing oxidative stress and protecting against ferroptosis-induced injury [120]. Another recent study demonstrated that activation of NRF2 resulted in increased expression of genes associated with telomere maintenance, including TERT. This enhanced telomerase activity promoted telomere lengthening, protecting against hypoxia-induced cardiomyocyte injury [121]. Moreover, NRF2 may influence telomere maintenance through the regulation of TERT and the pentose phosphate pathway, contributing to cell survival [122]. Therefore, it is plausible that, in the context of the aging process, a reduction in NRF2-driven TERT expression could play a key role in driving telomere attrition [13].
An age-related decline in the transcriptional activity of NRF2 and its associated genes may contribute to the lack of cellular resilience against oxidative stress and influence telomere dynamics. PRDX1, a target gene of NRF2, plays a critical role in maintaining redox balance, and its decline is associated with age-related changes in the cornea [123]. It has been demonstrated that PRDX1 acts as a protective factor, preventing oxidative damage to telomeres and ensuring the preservation of telomeric DNA integrity. By preserving telomeric DNA, PRDX1 facilitates the extension of telomeres by telomerase, which plays a crucial role in maintaining TL [72, 123]. Although the mechanism is not fully understood, NRF2 may interact with p53 and indirectly modulate telomere dynamics. It has been well established that p53 plays a critical role in maintaining telomere stability, preventing the accumulation of abnormal cells during aging. Mounting evidence suggests that mutant p53 may repress the elements of the NRF2-dependent oxidative stress response, attenuating the expression of phase 2 detoxifying enzymes and promoting the accumulation of ROS-induced damage. Given that p53 governs telomere regulation via TRF-2, a major determinant of TL, mutant p53 may induce telomere dysfunction and associated cellular responses (i.e., senescence and/or apoptosis) through inhibition of NRF2 pathway [124,125,126,127].
G-quadruplexes are secondary DNA structures that have gained significant attention due to their involvement in various biological processes, including aging and oxidative stress.
The multiple regulatory functions of G-quadruplexes have been shown not only to influence telomeres and telomerase but also to affect gene transcription and translation as a result of the presence of these motifs in gene promoters and untranslated regions (UTR) and open reading frames (ORF) of RNA molecules. G-quadruplex structures have been identified within the regulatory regions of NRF2, regulating its translation during oxidative stress. Although the exact regulatory behavior has not yet been elucidated, preliminary studies suggest that NRF2-mediated modulation of G-quadruplexes may contribute to TL regulation [128, 129]. In recent years, it has also emerged that one of the important functions of NRF2 is to modulate mitochondrial function. For example, there is a reciprocal regulatory loop between NRF2 and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), which is the best-known mitochondrial biogenesis mediator. Telomerase deficiency-induced downregulation of PGC1α and NRF2 was accompanied by mitochondrial dysfunction and disrupted oxidative metabolism [82, 130].
Targeting NRF2 to promote telomere maintenance
Targeting NRF2 activity and telomere maintenance mechanisms may represent an intriguing opportunity to treat the vast majority of age-related diseases. The most successful example of NRF2 modulators is dimethyl fumarate (DMF), currently the only NRF2 activator in clinical practice [131]. Activation of the NRF2 pathway by oral DMF administration alleviates oxidative stress and delays age-associated infertility in the mouse ovary. Likewise, after NRF2 activation through DMF, mRNA and protein levels of TERT, as well as mRNA amount of telomerase, were significantly elevated in the same animal model [132]. However, multiple compounds can theoretically restore NRF2 activity during aging and play a role in telomere maintenance, even in healthy individuals. Emerging evidence indicates that omega-3 (n-3) polyunsaturated fatty acids (PUFA), a large group of fatty acids with a broad spectrum of health benefits, may trigger a cytoprotective response via NRF2 activation [133]. Moreover, a recent meta-analysis of clinical trials suggests that n-3 PUFA, such as eicosapentaenoic acid (EPA; 20:5ω-3) and docosahexaenoic acid (DHA; 22:6ω-3), may potentially have clinical efficacy on telomere maintenance [14, 134]. Lifelong dietary DHA intervention enhanced the levels of NRF2 and profoundly attenuated telomere attrition, slowing the aging phenotype in telomerase-deficient mice [135].
The isothiocyanate sulforaphane is one of the most potent activators of NRF2. Many clinical trials have demonstrated that sulforaphane has the potential to prevent neoplastic effects through modulation of NRF2 activity, ameliorating a diversity of conditions characterized by oxidative and inflammatory stress [136, 137]. It has also been demonstrated that sulforaphane influences telomerase activity through epigenetic regulation by modulating histone acetylation levels and regulating DNA methyltransferases (DNMTs) activity in cancer lines [138, 139].
Cycloastragenol, a small molecule telomerase activator purified from the root of Astragalus membranaceus, has been shown to have beneficial effects on several conditions, including, insulin resistance, age-related macular degeneration, and CVD. Cycloastragenol not only enhances telomerase activity and elongates TL but also extends the healthspan and lifespan of mice without increasing the incidence of cancer [140]. Recently, it has been revealed that cycloastragenol functions at the intersection between NRF2, telomerase, and proteasome systems. The induction of telomerase activity by cycloastragenol is regulated by NRF2. However, this molecule not only increases the protein levels of human TERT but also its nuclear localization via upregulating heat shock protein 90 (Hsp90). In addition, by increasing NRF2 nuclear localization and activity, cycloastragenol upregulates cytoprotective enzymes and attenuates oxidative stress [141].
Curcumin, a lipophilic polyphenol compound derived from the rhizome of the turmeric plant, acts on multiple molecular targets that may play a protective role in various pathological conditions characterized by inflammation and increased oxidative stress [142]. For example, curcumin inhibits tumor progression through direct scavenging of ROS, induction of programmed cell death, and inhibition of NF-κB. Several studies have demonstrated the protective role of curcumin via NRF2 regulation, which in turn could maintain telomere integrity. Curcumin activates the NRF2 pathway and leads to the activation of antioxidant enzymes, including HO1, Hsp-70, TRX, and sirtuins [143,144,145]. As mentioned, the modulation of telomerase may be a promising approach for cancer treatment and anti-aging therapies. Based on the findings obtained from different studies, curcumin can both inhibit and maintain telomerase activity in a time- and dose-dependent manner. This effect is achieved through the regulation of human TERT translocation from the cytosol to the nucleus [146]. Quercetin and epigallocatechin gallate (EGCG) are other widely studied polyphenols that protect against oxidative damage through redox modulation of the NRF2 pathway. It has been reported that the antioxidant effect of these compounds may inhibit cardiac myocyte apoptosis by preventing telomere shortening and loss of TRF-2 expression. Similarly, low doses of other polyphenols, such as resveratrol, gallic acid, and kuromanin chloride, upregulate the mRNA levels of the human TERT in a hepatocellular carcinoma cell line, via induction of the NRF2 signaling pathway [147, 148].
Conclusions and future perspective
In recent years, significant advancements have been achieved in understanding the role of NRF2 in both health and disease. By restoring redox homeostasis and scavenging excessive ROS levels, NRF2 has become an attractive target to prevent and/or ameliorate several diseases associated with oxidative stress. There is growing evidence indicating that a significant decrease in NRF2 transcriptional activity plays a crucial role in the aging process, contributing to the onset and progression of age-related pathologies. An age-dependent decline of NRF2 and increased oxidative stress may affect cellular and molecular processes known to drive aging, including telomere shortening. TL has emerged as a key biomarker of aging and age-related diseases, with shorter telomeres being associated with increased mortality and morbidity. However, the measurement of TL as a biomarker of disease onset, disease progression, and mortality is still far from being used in clinical practice. Analytical issues and pathophysiological aspects, such as the lack of protocol standardization and accurate measurement of TL, are the primary barriers that impede the broader clinical application of TL measurement. As discussed in this review, the interplay between the NRF2 pathway and TL is complex and multifaceted, with evidence suggesting that NRF2 activation can promote telomere maintenance and delay cellular senescence. Nevertheless, further research is needed to fully elucidate the mechanisms underlying the relationship between the NRF2 pathway and TL. A promising area of research may involve the development of novel strategies that target both pathways simultaneously, to delay the onset of age-related diseases and improve healthspan. Although this field is still in its infancy, recent studies have highlighted compounds that possess the ability to target both the NRF2 pathway and telomere maintenance mechanisms. Future endeavors aimed at comprehending the intricate interplay between NRF2 and TL, alongside the identification of novel compounds concomitantly modulating these processes, will play a pivotal role in designing intervention strategies promoting healthy aging.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- 53BP1:
-
P53-binding protein
- 6PGD:
-
6-Phosphogluconate dehydrogenase
- 8-oxoG:
-
8-Oxoguanine
- AD:
-
Alzheimer’s disease
- AKR:
-
Aldo–keto reductase
- ALT:
-
Alternative lengthening of telomeres
- AMPK:
-
AMP-activated protein kinase
- AOX1:
-
Aldehyde oxidase
- ARE:
-
Antioxidant response elements
- ARG-1:
-
Arginase 1
- BER:
-
Base excision repair
- bZIP:
-
Basic leucine zipper
- CAT:
-
Catalase
- CES:
-
Carboxylesterases
- CNC:
-
Cap ‘n’ collar
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- Cul3:
-
Cullin 3
- CVD:
-
Cardiovascular disease
- DDR :
-
DNA damage response
- DHA:
-
Docosahexaenoic acid
- DMF:
-
Dimethyl fumarate
- DNMTs:
-
DNA methyltransferases
- EGCG:
-
Epigallocatechin gallate
- EPA:
-
Eicosapentaenoic acid
- FIZZ-1:
-
Found in inflammatory zone 1
- FTH:
-
Ferritin heavy chain
- FTL:
-
Ferritin light chain
- G6PD:
-
Glucose 6-phosphate dehydrogenase
- GCL:
-
γ-Glutamylcysteine synthetase
- GCLC:
-
Glutamate–cysteine ligase catalytic subunit
- GCLM:
-
Glutamate–cysteine ligase modifier subunit
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSR:
-
Glutathione reductase
- GST:
-
Glutathione S-transferases
- HIV:
-
Human immunodeficiency virus
- HO-1:
-
Heme oxygenase-1
- HR:
-
Homologous recombination
- Hsp90:
-
Heat shock protein 90
- ICAM-1:
-
Intercellular adhesion molecule-1
- IDH1:
-
Isocitrate dehydrogenase
- IDH1:
-
Isocitrate dehydrogenase 1
- IFN:
-
Interferon
- IL-10:
-
Interleukin 10
- IL-1β:
-
Interleukin-1-beta
- IL-4:
-
Interleukin 4
- IL-6:
-
Interleukin 6
- KEAP1:
-
Kelch-like ECH-associated protein 1
- lncRNA:
-
Long non-coding RNA
- MCP-1:
-
Monocyte chemoattractant protein-1
- ME1:
-
Malic enzyme 1
- MIP2:
-
Macrophage inflammatory protein-2
- MMSE:
-
Mini mental status exam
- MPTP:
-
Mitochondrial permeability transition pores
- MRP:
-
Multidrug resistant protein
- MT:
-
Metallothionein
- MTHFD2:
-
Methylenetetrahydrofolate dehydrogenase
- n-3:
-
Omega-3
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- Neh:
-
NRF2-ECH homology domain
- NF-κB:
-
Nuclear factor kappa B
- NHEJ:
-
Non-homologous end-joining
- NQO1:
-
NADPH quinone oxidoreductase 1
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- OATP2:
-
Organic anion transporting polypeptide 2
- ORF:
-
Open reading frames
- PD:
-
Parkinson’s disease
- PGC1α:
-
Peroxisome proliferator-activated receptor-γ coactivator 1α
- PGD:
-
Phosphogluconate dehydrogenase
- POT-1:
-
Protection of telomeres-1
- PPAT:
-
Phosphoribosyl pyrophosphate amidotransferase
- PRDX1:
-
Peroxiredoxin 1
- PUFA:
-
Polyunsaturated fatty acids
- RAD51:
-
RAD51 homolog 1
- RAP-1:
-
Repressor-activator protein 1
- RARα:
-
Retinoic acid receptor α
- RNP:
-
Ribonucleoprotein
- ROS:
-
Reactive oxygen species
- SASP:
-
Senescence-associated secretory phenotype
- SLC7A11:
-
Solute carrier family 7 member 11
- sMAF:
-
Small MAF proteins
- SOD:
-
Superoxide dismutase
- SOD1:
-
Cu–Zn superoxide dismutase
- SRXN:
-
Sulfiredoxin
- SRXN1:
-
Sulfiredoxin 1
- TERC:
-
Telomerase RNA component
- TERRA:
-
Telomeric repeat-containing RNA
- TERT:
-
Telomerase reverse transcriptase
- TIN-2:
-
TRF-1 interacting nuclear protein 2
- TL:
-
Telomere length
- TNF-α:
-
Tumor necrosis factor-alpha
- TPP-1:
-
TIN-2 and POT-1 interacting protein 1
- TRF-1:
-
Telomeric repeat binding factor 1
- TRF-2:
-
Telomeric repeat binding factor 2
- TRF-2:
-
Telomeric repeat binding factor 2
- TRX:
-
Thioredoxin
- TRXR:
-
Thioredoxin reductase
- UGT:
-
UDP-glucuronosyltransferase
- UPR:
-
Unfolded protein response
- UTR:
-
Untranslated regions
- XRCC5:
-
X-ray repair cross-complementing 5
- YBX1:
-
Y-box binding protein 1
- β-TrCP:
-
β-Transducin repeat-containing protein
References
López-Otín C, Blasco MA, Partridge L et al (2023) Hallmarks of aging: an expanding universe. Cell 186:243–278. https://doi.org/10.1016/J.CELL.2022.11.001
Richardson AG, Schadt EE (2014) The role of macromolecular damage in aging and age-related disease. J Gerontol A 69(Suppl 1):S28–S32. https://doi.org/10.1093/GERONA/GLU056
Ferrucci L, Gonzalez-Freire M, Fabbri E et al (2020) Measuring biological aging in humans: a quest. Aging Cell. https://doi.org/10.1111/ACEL.13080
Guo J, Huang X, Dou L et al (2022) Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. https://doi.org/10.1038/S41392-022-01251-0
Anik MI, Mahmud N, Al MA et al (2022) Role of reactive oxygen species in aging and age-related diseases: a review. ACS Appl Bio Mater. https://doi.org/10.1021/ACSABM.2C00411
Stefanatos R, Sanz A (2018) The role of mitochondrial ROS in the aging brain. FEBS Lett 592:743–758. https://doi.org/10.1002/1873-3468.12902
Wang L, Lu Z, Zhao J et al (2021) Selective oxidative stress induces dual damage to telomeres and mitochondria in human T cells. Aging Cell. https://doi.org/10.1111/ACEL.13513
Lee J, Li J, Johnson DA et al (2005) Nrf2, a multi-organ protector? FASEB J 19:1061–1066. https://doi.org/10.1096/FJ.04-2591HYP
Malhotra D, Portales-Casamar E, Singh A et al (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through chip-seq profiling and network analysis. Nucleic Acids Res 38:5718–5734. https://doi.org/10.1093/nar/gkq212
Davinelli S, Willcox DC, Scapagnini G (2012) Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun Ageing. https://doi.org/10.1186/1742-4933-9-9
Lewis KN, Wason E, Edrey YH et al (2015) Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci USA 112:3722–3727. https://doi.org/10.1073/PNAS.1417566112
Gureev AP, Shaforostova EA, Popov VN (2019) Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. https://doi.org/10.3389/FGENE.2019.00435/PDF
Schmidlin CJ, Dodson MB, Madhavan L, Zhang DD (2019) Redox regulation by NRF2 in aging and disease. Free Radic Biol Med 134:702–707. https://doi.org/10.1016/j.freeradbiomed.2019.01.016
Davinelli S, Medoro A, Intrieri M et al (2022) Targeting NRF2-KEAP1 axis by omega-3 fatty acids and their derivatives: emerging opportunities against aging and diseases. Free Radic Biol Med 193:736–750. https://doi.org/10.1016/J.FREERADBIOMED.2022.11.017
Fasching CL (2018) Telomere length measurement as a clinical biomarker of aging and disease. Crit Rev Clin Lab Sci 55:443–465. https://doi.org/10.1080/10408363.2018.1504274
Vaiserman A, Krasnienkov D (2021) Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front Genet. https://doi.org/10.3389/FGENE.2020.630186/PDF
Allsopp RC, Vaziri H, Patterson C et al (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 89:10114–10118. https://doi.org/10.1073/pnas.89.21.10114
Jacome Burbano MS, Cherfils‐Vicini J, Gilson E (2021) Neutrophils: mediating TelOxidation and senescence. EMBO J. https://doi.org/10.15252/EMBJ.2021108164
Chakravarti D, LaBella KA, DePinho RA (2021) Telomeres: history, health, and hallmarks of aging. Cell 184:306–322
Wang Z, Xiaoying WU (2021) Abnormal function of telomere protein TRF2 induces cell mutation and the effects of environmental tumor-promoting factors (review). Oncol Rep. https://doi.org/10.3892/OR.2021.8135/DOWNLOAD
Maher J, Yamamoto M (2010) The rise of antioxidant signaling—the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol 244:4–15. https://doi.org/10.1016/J.TAAP.2010.01.011
Francisqueti-Ferron FV, Ferron AJT, Garcia JL et al (2019) Basic concepts on the role of nuclear factor erythroid-derived 2-like 2 (Nrf2) in age-related diseases. Int J Mol Sci. https://doi.org/10.3390/IJMS20133208
Plafker KS, Nguyen L, Barneche M et al (2010) The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J Biol Chem 285:23064–23074. https://doi.org/10.1074/JBC.M110.121913
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29:1727–1745. https://doi.org/10.1089/ARS.2017.7342
Silva-Islas CA, Maldonado PD (2018) Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res 134:92–99. https://doi.org/10.1016/J.PHRS.2018.06.013
Mou Y, Wen S, Li YX et al (2020) Recent progress in Keap1-Nrf2 protein–protein interaction inhibitors. Eur J Med Chem. https://doi.org/10.1016/J.EJMECH.2020.112532
Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123:590–600. https://doi.org/10.1093/TOXSCI/KFR183
Kovac S, Angelova PR, Holmström KM et al (2015) Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta 1850:794–801. https://doi.org/10.1016/J.BBAGEN.2014.11.021
Harvey CJ, Thimmulappa RK, Singh A et al (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46:443–453. https://doi.org/10.1016/J.FREERADBIOMED.2008.10.040
Suh JH, Shenvi SV, Dixon BM et al (2004) Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA 101:3381–3386. https://doi.org/10.1073/PNAS.0400282101
Chen K, Wang S, Sun QW et al (2021) Klotho deficiency causes heart aging via impairing the Nrf2-GR pathway. Circ Res 128:492–507. https://doi.org/10.1161/CIRCRESAHA.120.317348
Yu C, Xiao JH (2021) The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev. https://doi.org/10.1155/2021/6635460
Zhang H, Davies KJA, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–336. https://doi.org/10.1016/J.FREERADBIOMED.2015.05.036
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023
Sivandzade F, Prasad S, Bhalerao A, Cucullo L (2019) NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. https://doi.org/10.1016/J.REDOX.2018.11.017
Davinelli S, Saso L, D’angeli F et al (2022) Astaxanthin as a modulator of Nrf2, NF-κB, and their crosstalk: molecular mechanisms and possible clinical applications. Molecules. https://doi.org/10.3390/MOLECULES27020502
Pan H, Wang H, Wang X et al (2012) The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm. https://doi.org/10.1155/2012/217580
Ahmed SMU, Luo L, Namani A et al (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta 1863:585–597
Kozakiewicz M, Kornatowski M, Krzywińska O, Kędziora-Kornatowska K (2019) Changes in the blood antioxidant defense of advanced age people. Clin Interv Aging 14:763–771. https://doi.org/10.2147/CIA.S201250
Pajares M, Rojo AI, Arias E et al (2018) Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy 14:1310–1322. https://doi.org/10.1080/15548627.2018.1474992
Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ et al (2016) Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 12:1902–1916. https://doi.org/10.1080/15548627.2016.1208889
Cullinan SB, Zhang D, Hannink M et al (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23:7198–7209. https://doi.org/10.1128/MCB.23.20.7198-7209.2003
Jayakumar S, Pal D, Sandur SK (2015) Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res 779:33–45. https://doi.org/10.1016/J.MRFMMM.2015.06.007
Kim SB, Pandita RK, Eskiocak U et al (2012) Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci USA. https://doi.org/10.1073/PNAS.1207718109/-/DCSUPPLEMENTAL/PNAS.201207718SI.PDF
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. https://doi.org/10.1016/J.FREERADBIOMED.2015.04.036
Yuan H, Xu Y, Luo Y et al (2021) Role of Nrf2 in cell senescence regulation. Mol Cell Biochem 476:247–259. https://doi.org/10.1007/S11010-020-03901-9
Joo MS, Kim WD, Lee KY et al (2016) AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol 36:1931–1942. https://doi.org/10.1128/MCB.00118-16
Sultana R, Perluigi M, Butterfield DA (2006) Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal 8:2021–2037. https://doi.org/10.1089/ARS.2006.8.2021
Ramsey CP, Glass CA, Montgomery MB et al (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66:75–85. https://doi.org/10.1097/NEN.0B013E31802D6DA9
Rojo AI, Pajares M, García-Yagüe AJ et al (2018) Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol 18:173–180. https://doi.org/10.1016/J.REDOX.2018.07.006
Jo C, Gundemir S, Pritchard S et al (2014) Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. https://doi.org/10.1038/NCOMMS4496
Gureev AP, Popov VN (2019) Nrf2/ARE pathway as a therapeutic target for the treatment of Parkinson diseases. Neurochem Res 44:2273–2279. https://doi.org/10.1007/S11064-018-02711-2
Lastres-Becker I, García-Yagüe AJ, Scannevin RH et al (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s disease. Antioxid Redox Signal 25:61–77. https://doi.org/10.1089/ARS.2015.6549
Wu S, Lu H, Bai Y (2019) Nrf2 in cancers: a double-edged sword. Cancer Med 8:2252–2267. https://doi.org/10.1002/CAM4.2101
Rojo de la Vega M, Chapman E, Zhang DD (2018) NRF2 and the hallmarks of cancer. Cancer Cell 34:21–43. https://doi.org/10.1016/J.CCELL.2018.03.022
Schmidlin CJ, Shakya A, Dodson M et al (2021) The intricacies of NRF2 regulation in cancer. Semin Cancer Biol 76:110–119. https://doi.org/10.1016/J.SEMCANCER.2021.05.016
Ali S, Corbi G, Medoro A et al (2023) Relationship between monounsaturated fatty acids and sarcopenia: a systematic review and meta-analysis of observational studies. Aging Clin Exp Res 35:1823–1834. https://doi.org/10.1007/S40520-023-02465-0
Wiedmer P, Jung T, Castro JP et al (2021) Sarcopenia—molecular mechanisms and open questions. Ageing Res Rev. https://doi.org/10.1016/J.ARR.2020.101200
Huang DD, Fan SD, Chen XY et al (2019) Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp Gerontol 119:61–73. https://doi.org/10.1016/J.EXGER.2019.01.022
Miller CJ, Gounder SS, Kannan S et al (2012) Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta 1822:1038–1050. https://doi.org/10.1016/J.BBADIS.2012.02.007
Kloska D, Kopacz A, Piechota-Polanczyk A et al (2019) Nrf2 in aging—focus on the cardiovascular system. Vasc Pharmacol 112:42–53. https://doi.org/10.1016/J.VPH.2018.08.009
D’Adda Di Fagagna F, Teo SH, Jackson SP (2004) Functional links between telomeres and proteins of the DNA-damage response. Genes Dev 18:1781–1799. https://doi.org/10.1101/GAD.1214504
De Lange T (2018) Shelterin-mediated telomere protection. Annu Rev Genet 52:223–247. https://doi.org/10.1146/ANNUREV-GENET-032918-021921
Maciejowski J, De Lange T (2017) Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 18:175–186. https://doi.org/10.1038/NRM.2016.171
Gala K, Khattar E (2021) Long non-coding RNAs at work on telomeres: functions and implications in cancer therapy. Cancer Lett 502:120–132. https://doi.org/10.1016/J.CANLET.2020.12.036
Shay JW, Wright WE (2019) Telomeres and telomerase: three decades of progress. Nat Rev Genet 20:299–309. https://doi.org/10.1038/S41576-019-0099-1
Muraki K, Nyhan K, Han L, Murnane JP (2012) Mechanisms of telomere loss and their consequences for chromosome instability. Front Oncol 2:1–13. https://doi.org/10.3389/fonc.2012.00135
Correia-Melo C, Hewitt G, Passos JF (2014) Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Health. https://doi.org/10.1186/2046-2395-3-1
Sfeir A, Kosiyatrakul ST, Hockemeyer D et al (2009) Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138:90–103. https://doi.org/10.1016/J.CELL.2009.06.021
De Rosa M, Johnson SA, Opresko PL (2021) Roles for the 8-oxoguanine DNA repair system in protecting telomeres from oxidative stress. Front Cell Dev Biol. https://doi.org/10.3389/FCELL.2021.758402/PDF
Fouquerel E, Barnes RP, Uttam S et al (2019) Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol Cell 75:117-130.e6. https://doi.org/10.1016/J.MOLCEL.2019.04.024
Aeby E, Ahmed W, Redon S et al (2016) Peroxiredoxin 1 protects telomeres from oxidative damage and preserves telomeric DNA for extension by telomerase. Cell Rep 17:3107–3114. https://doi.org/10.1016/J.CELREP.2016.11.071
Xu G, Herzig M, Rotrekl V, Walter CA (2008) Base excision repair, aging and health span. Mech Ageing Dev 129:366–382. https://doi.org/10.1016/J.MAD.2008.03.001
Oikawa S, Kawanishi S (1999) Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett 453:365–368. https://doi.org/10.1016/S0014-5793(99)00748-6
Opresko PL, Fan J, Danzy S et al (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res 33:1230–1239. https://doi.org/10.1093/NAR/GKI273
Karlseder J, Hoke K, Mirzoeva OK et al (2004) The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. https://doi.org/10.1371/JOURNAL.PBIO.0020240
Richter T, Saretzki G, Nelson G et al (2007) TRF2 overexpression diminishes repair of telomeric single-strand breaks and accelerates telomere shortening in human fibroblasts. Mech Ageing Dev 128:340–345. https://doi.org/10.1016/J.MAD.2007.02.003
Fouquerel E, Lormand J, Bose A et al (2016) Oxidative guanine base damage regulates human telomerase activity. Nat Struct Mol Biol 23:1092–1100. https://doi.org/10.1038/NSMB.3319
Lee HT, Bose A, Lee CY et al (2017) Molecular mechanisms by which oxidative DNA damage promotes telomerase activity. Nucleic Acids Res 45:11752–11765. https://doi.org/10.1093/NAR/GKX789
Barnes RP, Fouquerel E, Opresko PL (2019) The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177:37–45. https://doi.org/10.1016/J.MAD.2018.03.013
Ahmed W, Lingner J (2018) Impact of oxidative stress on telomere biology. Differentiation 99:21–27. https://doi.org/10.1016/J.DIFF.2017.12.002
Jurk D, Wilson C, Passos JF et al (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. https://doi.org/10.1038/NCOMMS5172
Shivappa N, Wirth MD, Hurley TG, Hébert JR (2017) Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol Nutr Food Res. https://doi.org/10.1002/MNFR.201600630
O’Donovan A, Pantell MS, Puterman E et al (2011) Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0019687
Amsellem V, Gary-Bobo G, Marcos E et al (2011) Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184:1358–1366. https://doi.org/10.1164/RCCM.201105-0802OC
Zhang J, Rane G, Dai X et al (2016) Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res Rev 25:55–69. https://doi.org/10.1016/J.ARR.2015.11.006
Cawthon RM, Smith KR, O’Brien E et al (2003) Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet (London, England) 361:393–395. https://doi.org/10.1016/S0140-6736(03)12384-7
Valdes AM, Richards JB, Gardner JP et al (2007) Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int 18:1203–1210. https://doi.org/10.1007/S00198-007-0357-5
Xu C, Wang Z, Su X et al (2020) Association between leucocyte telomere length and cardiovascular disease in a large general population in the United States. Sci Rep. https://doi.org/10.1038/S41598-019-57050-1
Shen G, Huang JY, Huang YQ, Feng YQ (2020) the relationship between telomere length and cancer mortality: data from the 1999–2002 National Healthy and Nutrition Examination Survey (NHANES). J Nutr Health Aging 24:9–15. https://doi.org/10.1007/S12603-019-1265-Z
Forero DA, González-Giraldo Y, López-Quintero C et al (2016) Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A 71:1069–1073. https://doi.org/10.1093/GERONA/GLW053
Demanelis K, Jasmine F, Chen LS et al (2020) Determinants of telomere length across human tissues. Science. https://doi.org/10.1126/SCIENCE.AAZ6876
Yu X, Liu MM, Zheng CY et al (2023) Telomerase reverse transcriptase and neurodegenerative diseases. Front Immunol 14:1165632. https://doi.org/10.3389/FIMMU.2023.1165632/PDF
Spilsbury A, Miwa S, Attems J, Saretzki G (2015) The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J Neurosci 35:1659–1674. https://doi.org/10.1523/JNEUROSCI.2925-14.2015
Tedone E, Arosio B, Colombo F et al (2015) Leukocyte telomere length in Alzheimer’s disease patients with a different rate of progression. J Alzheimers Dis 46:761–769. https://doi.org/10.3233/JAD-142808
Panossian LA, Porter VR, Valenzuela HF et al (2003) Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging 24:77–84. https://doi.org/10.1016/S0197-4580(02)00043-X
Fordyce CA, Heaphy CM, Bisoffi M et al (2006) Telomere content correlates with stage and prognosis in breast cancer. Breast Cancer Res Treat 99:193–202. https://doi.org/10.1007/S10549-006-9204-1
Heaphy CM, Gaonkar G, Peskoe SB et al (2015) Prostate stromal cell telomere shortening is associated with risk of prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Prostate 75:1160–1166. https://doi.org/10.1002/PROS.22997
Jia H, Wang Z (2016) Telomere length as a prognostic factor for overall survival in colorectal cancer patients. Cell Physiol Biochem 38:122–128. https://doi.org/10.1159/000438614
Hou L, Joyce BT, Gao T et al (2015) Blood telomere length attrition and cancer development in the normative aging study cohort. EBioMedicine 2:591–596. https://doi.org/10.1016/J.EBIOM.2015.04.008
Zhu X, Han W, Xue W et al (2016) The association between telomere length and cancer risk in population studies. Sci Rep. https://doi.org/10.1038/SREP22243
Weischer M, Nordestgaard BG, Cawthon RM et al (2013) Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 105:459–468. https://doi.org/10.1093/JNCI/DJT016
Julin B, Shui I, Heaphy CM et al (2015) Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer 112:769–776. https://doi.org/10.1038/BJC.2014.640
Haycock PC, Burgess S, Nounu A et al (2017) Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol 3:636–651. https://doi.org/10.1001/JAMAONCOL.2016.5945
DeBoy EA, Tassia MG, Schratz KE et al (2023) Familial clonal hematopoiesis in a long telomere syndrome. N Engl J Med 388:2422–2433. https://doi.org/10.1056/NEJMOA2300503
Sawhney V, Campbell NG, Brouilette SW et al (2016) Telomere shortening and telomerase activity in ischaemic cardiomyopathy patients—potential markers of ventricular arrhythmia. Int J Cardiol 207:157–163. https://doi.org/10.1016/J.IJCARD.2016.01.066
Brouilette SW, Moore JS, McMahon AD et al (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet (London, England) 369:107–114. https://doi.org/10.1016/S0140-6736(07)60071-3
Ogami M, Ikura Y, Ohsawa M et al (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24:546–550. https://doi.org/10.1161/01.ATV.0000117200.46938.E7
Sharifi-Sanjani M, Oyster NM, Tichy ED et al (2017) Cardiomyocyte-specific telomere shortening is a distinct signature of heart failure in humans. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.005086
Carty CL, Kooperberg C, Liu J et al (2015) Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol 35:2225–2231. https://doi.org/10.1161/ATVBAHA.115.305838
Calvert PA, Liew TV, Gorenne I et al (2011) Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol 31:2157–2164. https://doi.org/10.1161/ATVBAHA.111.229237
Gruber HJ, Semeraro MD, Renner W, Herrmann M (2021) Telomeres and age-related diseases. Biomedicines. https://doi.org/10.3390/BIOMEDICINES9101335
Wang J, Dong X, Cao L et al (2016) Association between telomere length and diabetes mellitus: a meta-analysis. J Int Med Res 44:1156–1173. https://doi.org/10.1177/0300060516667132
Sanders JL, Cauley JA, Boudreau RM et al (2009) Leukocyte telomere length is not associated with BMD, osteoporosis, or fracture in older adults: results from the health, aging and body composition study. J Bone Miner Res 24:1531–1536. https://doi.org/10.1359/JBMR.090318
Davinelli S, Scapagnini G, Denaro F et al (2014) Altered expression pattern of Nrf2/HO-1 axis during accelerated-senescence in HIV-1 transgenic rat. Biogerontology. https://doi.org/10.1007/s10522-014-9511-6
Singh MV, Kotla S, Le NT et al (2019) Senescent phenotype induced by p90RSK-NRF2 signaling sensitizes monocytes and macrophages to oxidative stress in HIV-positive individuals. Circulation 139:1199–1216. https://doi.org/10.1161/CIRCULATIONAHA.118.036232
Hu J, Hwang SS, Liesa M et al (2012) Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell 148:651–663. https://doi.org/10.1016/J.CELL.2011.12.028
Gong C, Yang H, Wang S et al (2021) hTERT promotes CRC proliferation and migration by recruiting YBX1 to increase NRF2 expression. Front Cell Dev Biol. https://doi.org/10.3389/FCELL.2021.658101/PDF
Liu T, Long Q, Li L et al (2021) The NRF2-dependent transcriptional axis, XRCC5/hTERT drives tumor progression and 5-Fu insensitivity in hepatocellular carcinoma. Mol Ther Oncolytics 24:249–261. https://doi.org/10.1016/J.OMTO.2021.12.012
Dong H, Xia Y, Jin S et al (2021) Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. https://doi.org/10.1038/S41419-021-04307-1
Wu W, Du Z, Wu L (2022) Dexmedetomidine attenuates hypoxia-induced cardiomyocyte injury by promoting telomere/telomerase activity: possible involvement of ERK1/2-Nrf2 signaling pathway. Cell Biol Int 46:1036–1046. https://doi.org/10.1002/CBIN.11799
Ahmad F, Dixit D, Sharma V et al (2016) Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. https://doi.org/10.1038/CDDIS.2016.117
Lovatt M, Adnan K, Kocaba V et al (2020) Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol. https://doi.org/10.1016/J.REDOX.2019.101417
Begus-Nahrmann Y, Lechel A, Obenauf AC et al (2009) p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet 41:1138–1143. https://doi.org/10.1038/NG.426
Kalo E, Kogan-Sakin I, Solomon H et al (2012) Mutant p53R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen species. J Cell Sci 125:5578–5586. https://doi.org/10.1242/JCS.106815
Chen D, Tavana O, Chu B et al (2017) NRF2 is a major target of ARF in p53-independent tumor suppression. Mol Cell 68:224-232.e4. https://doi.org/10.1016/J.MOLCEL.2017.09.009
D’Adda Di Fagagna F, Reaper PM, Clay-Farrace L et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198. https://doi.org/10.1038/NATURE02118
Lee SC, Zhang J, Strom J et al (2016) G-Quadruplex in the NRF2 mRNA 5′ untranslated region regulates de novo NRF2 protein translation under oxidative stress. Mol Cell Biol. https://doi.org/10.1128/MCB.00122-16
Tian T, Chen YQ, Wang SR, Zhou X (2018) G-quadruplex: a regulator of gene expression and its chemical targeting. Chem 4:1314–1344. https://doi.org/10.1016/J.CHEMPR.2018.02.014
Xiong S, Patrushev N, Forouzandeh F et al (2015) PGC-1α modulates telomere function and DNA damage in protecting against aging-related chronic diseases. Cell Rep 12:1391–1399. https://doi.org/10.1016/J.CELREP.2015.07.047
Dinkova-Kostova AT, Copple IM (2023) Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol Sci 44:137–149. https://doi.org/10.1016/J.TIPS.2022.12.003
Akino N, Wada-Hiraike O, Isono W et al (2019) Activation of Nrf2/Keap1 pathway by oral dimethylfumarate administration alleviates oxidative stress and age-associated infertility might be delayed in the mouse ovary. Reprod Biol Endocrinol. https://doi.org/10.1186/S12958-019-0466-Y
Abrescia P, Treppiccione L, Rossi M, Bergamo P (2020) Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog Lipid Res. https://doi.org/10.1016/J.PLIPRES.2020.101066
Ali S, Scapagnini G, Davinelli S (2022) Effect of omega-3 fatty acids on the telomere length: a mini meta-analysis of clinical trials. Biomol Concepts 13:25–33. https://doi.org/10.1515/BMC-2021-0024/PDF
Wu S, Wu Y, Chen J et al (2023) Lifelong docosahexaenoic acid intervention ameliorates aging in the telomere-DNA-mitochondria axis in telomerase-deficient mice. J Nutr Biochem. https://doi.org/10.1016/J.JNUTBIO.2022.109202
Yagishita Y, Gatbonton-schwager TN, McCallum ML, Kensler TW (2020) Current landscape of NRF2 biomarkers in clinical trials. Antioxidants (Basel, Switzerland) 9:1–36. https://doi.org/10.3390/ANTIOX9080716
Yagishita Y, Fahey JW, Dinkova-Kostova AT, Kensler TW (2019) Broccoli or sulforaphane: is it the source or dose that matters? Molecules. https://doi.org/10.3390/MOLECULES24193593
Abbas A, Hall JA, Patterson WL et al (2016) Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem Cell Biol 94:71–81. https://doi.org/10.1139/BCB-2015-0038
Meeran SM, Patel SN, Tollefsbol TO (2010) Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0011457
de Jesus BB, Schneeberger K, Vera E et al (2011) The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10:604–621. https://doi.org/10.1111/J.1474-9726.2011.00700.X
Yilmaz S, Bedir E, Ballar Kirmizibayrak P (2022) The role of cycloastragenol at the intersection of NRF2/ARE, telomerase, and proteasome activity. Free Radic Biol Med 188:105–116. https://doi.org/10.1016/J.FREERADBIOMED.2022.06.230
Kunnumakkara AB, Bordoloi D, Padmavathi G et al (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174:1325–1348. https://doi.org/10.1111/BPH.13621
Liczbiński P, Michałowicz J, Bukowska B (2020) Molecular mechanism of curcumin action in signaling pathways: review of the latest research. Phytother Res 34:1992–2005. https://doi.org/10.1002/PTR.6663
Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S (2021) The role of curcumin in aging and senescence: molecular mechanisms. Biomed Pharmacother. https://doi.org/10.1016/J.BIOPHA.2020.111119
Sorrenti V, Buriani A, Fortinguerra S et al (2023) Cell survival, death, and proliferation in senescent and cancer cells: the role of (poly)phenols. Adv Nutr. https://doi.org/10.1016/J.ADVNUT.2023.05.014
Forouzanfar F, Majeed M, Jamialahmadi T, Sahebkar A (2021) Telomerase: a target for therapeutic effects of curcumin in cancer. Adv Exp Med Biol 1286:135–143. https://doi.org/10.1007/978-3-030-55035-6_10
Sheng R, Gu ZL, Xie ML (2013) Epigallocatechin gallate, the major component of polyphenols in green tea, inhibits telomere attrition mediated cardiomyocyte apoptosis in cardiac hypertrophy. Int J Cardiol 162:199–209. https://doi.org/10.1016/J.IJCARD.2011.07.083
Moghadam D, Zarei R, Vakili S et al (2023) The effect of natural polyphenols resveratrol, gallic acid, and kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol Biol Rep 50:77–84. https://doi.org/10.1007/S11033-022-08031-7
Acknowledgements
None.
Funding
Open access funding provided by Università degli Studi del Molise within the CRUI-CARE Agreement. No specific funding was received for this manuscript.
Author information
Authors and Affiliations
Contributions
AM and SD conceptualized the review. AM and SD performed extensive literature review. SD wrote the first draft of the manuscript. AM prepared figures and tables. AM, LS, GS, and SD contributed to critically reviewing the manuscript, revising it for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All the authors have read the manuscript and consented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Medoro, A., Saso, L., Scapagnini, G. et al. NRF2 signaling pathway and telomere length in aging and age-related diseases. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04878-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04878-x