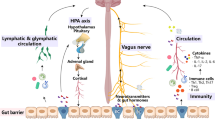
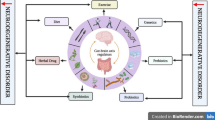
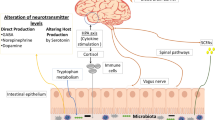
Abstract
There are complex interactions between the gut and the brain. With increasing research on the relationship between gut microbiota and brain function, accumulated clinical and preclinical evidence suggests that gut microbiota is intimately involved in the pathogenesis of neurodegenerative diseases (NDs). Increasingly studies are beginning to focus on the association between gut microbiota and central nervous system (CNS) degenerative pathologies to find potential therapies for these refractory diseases. In this review, we summarize the changes in the gut microbiota in Alzheimer's disease, Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis and contribute to our understanding of the function of the gut microbiota in NDs and its possible involvement in the pathogenesis. We subsequently discuss therapeutic approaches targeting gut microbial abnormalities in these diseases, including antibiotics, diet, probiotics, and fecal microbiota transplantation (FMT). Furthermore, we summarize some completed and ongoing clinical trials of interventions with gut microbes for NDs, which may provide new ideas for studying NDs.

Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- MGB:
-
Microbiota–gut–brain
- NDs:
-
Neurodegenerative diseases
- CNS:
-
Central nervous system
- AD:
-
Alzheimer's disease
- PD:
-
Parkinson's disease
- MS:
-
Multiple sclerosis
- ALS:
-
Amyotrophic lateral sclerosis
- Aβ:
-
β-Amyloid
- NFTs:
-
Neurogenic fiber tangles
- MCI:
-
Mild cognitive impairment
- HC:
-
Healthy controls
- NPS:
-
Neuropsychiatric symptoms
- SCFAs:
-
Short-chain fatty acids
- NPS:
-
Neuropsychiatric symptoms
- ADLPAPT :
-
Amyloid and neurofibrillary tangles
- WT:
-
Wild type
- EECs:
-
Intestinal endocrine cells
- APPPS1:
-
Amyloidosis mouse of the AD model
- TLR4:
-
Toll-like receptor 4
- α-Syn:
-
α-Synuclein
- DMX:
-
Dorsal motor nucleus of the vagus nerve
- RRMS:
-
Relapsing–remitting MS
- FMT:
-
Fecal microbial transplantation
- 6-OHDA:
-
6-Hydroxydopamine
- EAE:
-
Experimental autoimmune encephalomyelitis
- KD:
-
Ketogenic diet
- APP/PS1:
-
APPswe/PS1dE9
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- IL-1:
-
Interleukin-1
- IFN-γ:
-
Interferon-γ
- IL-4:
-
Interleukin-4
- IL-10:
-
Interleukin-10
- 5-HT:
-
5-Hydroxytryptamine
- GABA:
-
γ-Aminobutyric acid
- Glu:
-
Glutamic acid
- CCK:
-
Chole cystokinin
- YYP:
-
YY peptide
- GRF:
-
Glucagon-releasing factor
- GLP-1:
-
Glucagon-like peptide-1
- Trp:
-
Tryptophan
- Tyr:
-
Tyrosine
- Gln:
-
Glutamine
References
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155
Belkaid Y, Harrison OJ (2017) Homeostatic Immunity and the Microbiota. Immunity 46:562–576
Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L et al (2018) Microbiota and metabolic diseases. Endocrine 61:357–371
Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK (2019) Microbiome-microglia connections via the gut-brain axis. J Exp Med 216:41–59
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65
Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E (2017) The gut microbiome in human neurological disease: A review. Ann Neurol 81:369–382
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19:55–71
Sampson TR, Mazmanian SK (2015) Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17:565–576
Cenit MC, Sanz Y, Codoñer-Franch P (2017) Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol 23:5486–5498
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15:565–581
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. The Lancet Neurology 19:179–194
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS et al (2019) The Microbiota-Gut-Brain Axis. Physiol Rev 99:1877–2013
Powell N, Walker MM, Talley NJ (2017) The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol 14:143–159
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516
Varatharaj A, Galea I (2017) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12
Osadchiy V, Martin CR, Mayer EA (2019) The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol 17:322–332
Browning KN, Verheijden S, Boeckxstaens GE (2017) The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology 152:730–744
Bonaz B, Sinniger V, Pellissier S (2017) The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front Immunol 8:1452
Cawthon CR, de La Serre CB (2018) Gut bacteria interaction with vagal afferents. Brain Res 1693:134–139
Pavlov VA, Tracey KJ (2015) Neural circuitry and immunity. Immunol Res 63:38–57
Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F (2020) Gut Microbiota Regulates Depression-Like Behavior in Rats Through the Neuroendocrine-Immune-Mitochondrial Pathway. Neuropsychiatr Dis Treat 16:859–869
Chen Z, Trapp BD (2016) Microglia and neuroprotection. J Neurochem 136(Suppl 1):10–17
Wenzel TJ, Gates EJ, Ranger AL, Klegeris A (2020) Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci 105:103493
Cussotto S, Sandhu KV, Dinan TG, Cryan JF (2018) The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front Neuroendocrinol 51:80–101
Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR et al (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb j 29:1395–1403
Gao K, Mu CL, Farzi A, Zhu WY (2020) Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr 11:709–723
Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E et al (2013) Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci 7:9
Gorjifard S, Goldszmid RS (2016) Microbiota-myeloid cell crosstalk beyond the gut. J Leukoc Biol 100:865–879
Lach G, Schellekens H, Dinan TG, Cryan JF (2018) Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 15:36–59
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK (2016) The Central Nervous System and the Gut Microbiome. Cell 167:915–932
Dinan TG, Cryan JF (2017) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595:489–503
Weidner WS, Barbarino P (2019) THE STATE OF THE ART OF DEMENTIA RESEARCH: NEW FRONTIERS. Alzheimers Dement 15:P1473
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine 14:5541–5554
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32
Pereira CF, Santos AE, Moreira PI, Pereira AC, Sousa FJ et al (2019) Is Alzheimer’s disease an inflammasomopathy? Ageing Res Rev 56:100966
Wu KM, Zhang YR, Huang YY, Dong Q, Tan L, Yu JT (2021) The role of the immune system in Alzheimer’s disease. Ageing Res Rev 70:101409
Bradburn S, Murgatroyd C, Ray N (2019) Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res Rev 50:1–8
Qian XH, Song XX, Liu XL, Chen SD, Tang HD (2021) Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res Rev 68:101317
van Olst L, Roks SJM, Kamermans A, Verhaar BJH, van der Geest AM et al (2021) Contribution of Gut Microbiota to Immunological Changes in Alzheimer’s Disease. Front Immunol 12:683068
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N et al (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68
Liu P, Wu L, Peng G, Han Y, Tang R et al (2019) Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 80:633–643
Zhou Y, Wang Y, Quan M, Zhao H, Jia J (2021) Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 81:583–595
Kim MS, Kim Y, Choi H, Kim W, Park S et al (2020) Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69:283–294
Miller AL, Bessho S, Grando K, Tükel Ç (2021) Microbiome or Infections: Amyloid-Containing Biofilms as a Trigger for Complex Human Diseases. Front Immunol 12:638867
Honarpisheh P, Reynolds CR, Blasco Conesa MP, Moruno Manchon JF, Putluri N et al (2020) Dysregulated gut homeostasis observed prior to the accumulation of the brain amyloid-β in Tg2576 mice. Int J Mol Sci 21(5):1711
Colombo AV, Sadler RK, Llovera G, Singh V, Roth S et al (2021) Microbiota-derived short chain fatty acids modulate microglia and promote Abeta plaque deposition. Elife 10:e59826
Cui L, Hou NN, Wu HM, Zuo X, Lian YZ et al (2020) Prevalence of Alzheimer’s Disease and Parkinson’s Disease in China: An Updated Systematical Analysis. Front Aging Neurosci 12:603854
Rossi A, Berger K, Chen H, Leslie D, Mailman RB, Huang X (2018) Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov Disord 33:156–159
Marras C, Beck JC, Bower JH, Roberts E, Ritz B et al (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis 4:21
Kalia LV, Lang AE (2015) Parkinson’s disease. The Lancet 386:896–912
Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M et al (2018) Does Parkinson’s disease start in the gut? Acta Neuropathol 135:1–12
Lai F, Jiang R, Xie W, Liu X, Tang Y et al (2018) Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem Res 43:1986–1999
Lubomski M, Davis RL, Sue CM (2020) Gastrointestinal dysfunction in Parkinson’s disease. J Neurol 267:1377–1388
Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A (2021) Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis 7:27
Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16:129
Vascellari S, Palmas V, Melis M, Pisanu S, Cusano R et al (2020) Gut microbiota and metabolome alterations associated with Parkinson’s disease. mSystems 5(5):e00561–20
Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 22:657–673
Dumitrescu L, Marta D, Dănău A, Lefter A, Tulbă D et al (2021) Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front Neurosci 15:689723
Chen X, Feng W, Ou R, Liu J, Yang J et al (2021) Evidence for Peripheral Immune Activation in Parkinson’s Disease. Front Aging Neurosci 13:617370
Reichardt F, Chassaing B, Nezami BG, Li G, Tabatabavakili S et al (2017) Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J Physiol 595:1831–1846
Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM et al (2019) Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68:829–843
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L et al (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF et al (2017) Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 88:1996–2002
Killinger BA, Madaj Z, Sikora JW, Rey N, Haas AJ et al (2018) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10(465):eaar5280
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W et al (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820
Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, Takahashi R (2018) Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener 13:21
Li Y, Chen Y, Jiang L, Zhang J, Tong X et al (2021) Intestinal Inflammation and Parkinson’s Disease. Aging Dis 12:2052–2068
Hirayama M, Ohno K (2021) Parkinson’s Disease and Gut Microbiota. Ann Nutr Metab 77(Suppl 2):28–35
Rani L, Mondal AC (2021) Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci Res 168:100–112
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A et al (2018) Multiple sclerosis Nat Rev Dis Primers 4:43
Ochoa-Repáraz J, Kasper LH (2014) Gut microbiome and the risk factors in central nervous system autoimmunity. FEBS Lett 588:4214–4222
Shahi SK, Freedman SN, Mangalam AK (2017) Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 8:607–615
Akdis CA (2021) Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol 21(11):739–751
Jangi S, Gandhi R, Cox LM, Li N, von Glehn F et al (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:12015
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S et al (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 114:10713–10718
Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH et al (2021) Gut Microbiome in Progressive Multiple Sclerosis. Ann Neurol 89:1195–1211
Touil H, Kobert A, Lebeurrier N, Rieger A, Saikali P et al (2018) Human central nervous system astrocytes support survival and activation of B cells: implications for MS pathogenesis. J Neuroinflammation 15:114
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597
Haase S, Haghikia A, Wilck N, Müller DN, Linker RA (2018) Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 154:230–238
Shen J, Yang L, You K, Chen T, Su Z et al (2022) Indole-3-Acetic Acid Alters Intestinal Microbiota and Alleviates Ankylosing Spondylitis in Mice. Front Immunol 13:762580
Wang X, Liang Z, Wang S, Ma D, Zhu M, Feng J (2022) Role of gut microbiota in multiple sclerosis and potential therapeutic implications. Curr Neuropharmacol 20(7):1413–1426
Niedermeyer S, Murn M, Choi PJ (2019) Respiratory Failure in Amyotrophic Lateral Sclerosis. Chest 155:401–408
Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3:17071
Wang MD, Little J, Gomes J, Cashman NR, Krewski D (2017) Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61:101–130
Weiss GA, Hennet T (2017) Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci 74:2959–2977
Milani C, Duranti S, Bottacini F, Casey E, Turroni F et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81(4):e00036–17
Boddy SL, Giovannelli I, Sassani M, Cooper-Knock J, Snyder MP et al (2021) The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med 19:13
Mazzini L, Mogna L, De Marchi F, Amoruso A, Pane M et al (2018) Potential Role of Gut Microbiota in ALS Pathogenesis and Possible Novel Therapeutic Strategies. J Clin Gastroenterol 2017:68–70
Zeng Q, Shen J, Chen K, Zhou J, Liao Q et al (2020) The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci Rep 10:12998
Burberry A, Wells MF, Limone F, Couto A, Smith KS et al (2020) C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582:89–94
Liu H, Wang J, He T, Becker S, Zhang G et al (2018) Butyrate: A Double-Edged Sword for Health? Adv Nutr 9:21–29
Nicholson K, Bjornevik K, Abu-Ali G, Chan J, Cortese M et al (2021) The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 22:186–194
Zhang YG, Wu S, Yi J, Xia Y, Jin D et al (2017) Target Intestinal Microbiota to Alleviate Disease Progression in Amyotrophic Lateral Sclerosis. Clin Ther 39:322–336
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U et al (2019) Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572:474–480
Surana NK (2019) Harnessing the microbiota to treat neurological diseases. Dialogues Clin Neurosci 21(2):159–165
Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19(2):179–194
Griffiths JA, Mazmanian SK (2018) Emerging evidence linking the gut microbiome to neurologic disorders. Genome Med 10:98
Ianiro G, Tilg H, Gasbarrini A (2016) Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65:1906
Becattini S, Taur Y, Pamer EG (2016) Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol Med 22:458–478
Garrido‐Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169(2):337–352
Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E et al (2017) Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun 8:15062
Umeda T, Hatanaka Y, Sakai A, Tomiyama T (2021) Nasal rifampicin improves cognition in a mouse model of dementia with Lewy bodies by reducing α-Synuclein oligomers. Int J Mol Sci 22(16):8453
Wu LH, Huang BR, Lai SW, Lin C, Lin HY et al (2020) SIRT1 activation by minocycline on regulation of microglial polarization homeostasis. Aging (Albany NY) 12:17990–18007
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X et al (2016) Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6:30028
Medina L, González-Lizárraga F, Dominguez-Meijide A, Ploper D, Parrales V et al (2021) Doxycycline Interferes With Tau Aggregation and Reduces Its Neuronal Toxicity. Front Aging Neurosci 13:635760
Liu H, Su W, Li S, Du W, Ma X et al (2017) Eradication of Helicobacter pylori infection might improve clinical status of patients with Parkinson’s disease, especially on bradykinesia. Clin Neurol Neurosurg 160:101
Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL (2010) Helicobacter pylori infection and motor fluctuations in patients with Parkinson’s disease. Mov Disord 23:1696–1700
Koutzoumis DN, Vergara M, Pino J, Buddendorff J, Khoshbouei H et al (2020) Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp Neurol 325:113159
Colpitts SL, Kasper EJ, Keever A, Liljenberg C, Kirby T et al (2017) A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 8:561–573
Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S (2019) Butyrate suppresses demyelination and enhances remyelination. J Neuroinflammation 16:165
Obrenovich M, Jaworski H, Tadimalla T, Mistry A, Sykes L et al (2020) The role of the microbiota–gut–brain axis and antibiotics in ALS and neurodegenerative diseases. Microorganisms 8(5):784
Santa-Cecília FV, Leite CA, Del-Bel E, Raisman-Vozari R (2019) The Neuroprotective Effect of Doxycycline on Neurodegenerative Diseases. Neurotox Res 35:981–986
Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Cammarota G (2016) The role of diet on gut microbiota composition. Eur Rev Med Pharmacol 20:4742–4749
Fernandez-Sanz P, Ruiz-Gabarre D, Garcia-Escudero V (2019) Modulating effect of diet on alzheimer’s disease. Diseases 7(1):12
Grosso G (2021) Nutritional psychiatry: how diet affects brain through gut microbiota. Nutrients 13(4):1282
Zhu TB, Zhang Z, Luo P, Wang SS, Peng Y et al (2019) Lipid metabolism in Alzheimer’s disease. Brain Res Bull 144:68–74
Tan BL, Norhaizan ME (2019) Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11(11):2579
Yazici D, Sezer H (2017) Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol 960:277–304
Lin B, Hasegawa Y, Takane K, Koibuchi N, Cao C, Kim-Mitsuyama S (2016) High‐fat‐diet intake enhances cerebral amyloid angiopathy and cognitive impairment in a mouse model of Alzheimer’s disease, independently of metabolic disorders. J Am Heart Assoc 5(6):e003154
Mirabelli M, Chiefari E, Arcidiacono B, Corigliano DM, Brunetti FS et al (2020) Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients 12(4):1066
Diolintzi A, Panagiotakos DB, Sidossis LS (2019) From Mediterranean diet to Mediterranean lifestyle: a narrative review. Public Health Nutr 22:2703–2713
Davis C, Bryan J, Hodgson J, Murphy K (2015) Definition of the Mediterranean Diet; a Literature Review. Nutrients 7:9139–9153
de la Rubia Ortí JE, García-Pardo MP, Drehmer E, Sancho Cantus D, Julián Rochina M et al (2018) Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J Alzheimers Dis 65:577–587
Paknahad Z, Sheklabadi E, Derakhshan Y, Bagherniya M, Chitsaz A (2020) The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement Ther Med 50:102366
Kraeuter AK, Phillips R, Sarnyai Z (2020) Ketogenic therapy in neurodegenerative and psychiatric disorders: From mice to men. Prog Neuropsychopharmacol Biol Psychiatry 101:109913
Bough KJ, Rho JM (2007) Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48:43–58
Phillips MCL, Deprez LM, Mortimer GMN, Murtagh DKJ, McCoy S et al (2021) Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res Ther 13:51
Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP (2018) Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov Disord 33:1306–1314
Choi IY, Piccio L, Childress P, Bollman B, Ghosh A et al (2016) A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep 15:2136–2146
Ludolph AC, Dorst J, Dreyhaupt J, Weishaupt JH, Kassubek J et al (2020) Effect of High-Caloric Nutrition on Survival in Amyotrophic Lateral Sclerosis. Ann Neurol 87:206–216
Fakhri S, Yarmohammadi A, Yarmohammadi M, Farzaei MH, Echeverria J (2021) Marine Natural Products: Promising Candidates in the Modulation of Gut-Brain Axis towards Neuroprotection. Mar Drugs 19:165
Zhang TT, Xu J, Wang YM, Xue CH (2019) Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog Lipid Res 75:100997
Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF (2020) Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol 60:477–502
Zhang CX, Wang HY, Chen TX (2019) Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed Res Int 2019:6764919
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Francavilla R (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ et al (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G et al (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17:687–701
Li C, Niu Z, Zou M, Liu S, Wang M et al (2020) Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J Dairy Sci 103:5816–5829
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(9):1021
Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F et al (2019) Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr 38:2569–2575
Sun J, Xu J, Yang B, Chen K, Kong Y et al (2020) Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol Nutr Food Res 64:e1900636
Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS et al (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7:2426
Yang X, Yu D, Xue L, Li H, Du J (2020) Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B 10:475–487
Castelli V, d’Angelo M, Lombardi F, Alfonsetti M, Antonosante A et al (2020) Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging (Albany NY) 12:4641–4659
Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F et al (2019) Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr 38:1031–1035
Atabati H, Yazdanpanah E, Mortazavi H, Bajestani SG, Raoofi A et al (2021) Immunoregulatory Effects of Tolerogenic Probiotics in Multiple Sclerosis. Adv Exp Med Biol 1286:87–105
Hutchinson AN, Bergh C, Kruger K, Sűsserová M, Allen J et al (2021) The effect of probiotics on health outcomes in the elderly: a systematic review of randomized, placebo-controlled studies. Microorganisms 9(6):1344
Tamtaji OR, Kouchaki E, Salami M, Aghadavod E, Akbari E et al (2017) The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin, and Lipids in Patients With Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Coll Nutr 36:660–665
Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R et al (2017) Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin Nutr 36:1245–1249
Tankou SK, Regev K, Healy BC, Tjon E, Laghi L et al (2018) A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 83:1147–1161
Calvo-Barreiro L, Eixarch H, Ponce-Alonso M, Castillo M, Lebrón-Galán R et al (2020) A commercial probiotic induces tolerogenic and reduces pathogenic responses in experimental autoimmune encephalomyelitis. Cells 9(4):906
Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF et al (2019) Next generation probiotics in disease amelioration. J Food Drug Anal 27:615–622
Tekin R. Fecal Microbiota Transplantation. Proc EKMUD 2015, 2015
Du H, Kuang TT, Qiu S, Xu T, Gang Huan CL et al (2019) Fecal medicines used in traditional medical system of China: a systematic review of their names, original species, traditional uses, and modern investigations. Chin Med 14:31
Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A et al (2015) Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Ann Intern Med 162:630–638
Weingarden AR, Vaughn BP (2017) Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 8:238–252
Gupta A, Saha S, Khanna S (2020) Therapies to modulate gut microbiota: Past, present and future. World J Gastroenterol 26:777–788
Kang Y, Kang X, Zhang H, Liu Q, Yang H, Fan W (2021) Gut Microbiota and Parkinson’s Disease: Implications for Faecal Microbiota Transplantation Therapy. ASN Neuro 13:17590914211016216
Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD et al (2018) Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70:48–60
Kuai XY, Yao XH, Xu LJ, Zhou YQ, Zhang LP et al (2021) Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Fact 20:98
Kim MS, Kim Y, Choi H, Kim W, Mook-Jung I (2019) Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 69:2018–317431
Wang H, Yang F, Xin R, Cui D, Sun Y (2020) The gut microbiota attenuate neuroinflammation in manganese exposure by inhibiting cerebral NLRP3 inflammasome. Biomed Pharmacother 129:110449
Sun J, Xu J, Ling Y, Wang F, Gong T et al (2019) Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry 9:189
Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM et al (2019) Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe 26(779–94):e8
Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW et al (2020) Fecal Microbiota Transplantation in Neurological Disorders. Front Cell Infect Microbiol 10:98
Mandrioli J, Amedei A, Cammarota G, Niccolai E, Zucchi E et al (2019) FETR-ALS Study Protocol: A Randomized Clinical Trial of Fecal Microbiota Transplantation in Amyotrophic Lateral Sclerosis. Front Neurol 10:1021
Green JE, Davis JA, Berk M, Hair C, Loughman A et al (2020) Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes 12:1–25
Cammarota G, Ianiro G, Bibbò S, Gas Ba Rrini A (2014) Gut microbiota modulation: probiotics, antibiotics or fecal microbiota transplantation? Int Emerg Med 9(4):365–373
Hardt S (2008) The role of microbiota in infectious disease. Trends Microbiol 16(3):107–114
DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA et al (2019) Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med 381:2043–2050
Smirnov KS, Maier TV, Walker A, Heinzmann SS, Forcisi S et al (2016) Challenges of metabolomics in human gut microbiota research. Int J Med Microbiol 306:266–279
Arnold JW, Roach J, Azcarate-Peril MA (2016) Emerging Technologies for Gut Microbiome Research. Trends Microbiol 24:887–901
Watterson WJ, Tanyeri M, Watson AR, Cham CM, Tay S (2020) Droplet-based high-throughput cultivation for accurate screening of antibiotic resistant gut microbes. ELife 9:e56998
Luan H, Wang X, Cai Z (2019) Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom Rev 38:22–33
Funding
This work was supported by the National Natural Science Foundation of China (No. 81973626, No. 81774059), the Tianjin Municipal Science and Technology Commission of China (No. 21JCYBJC01620), Tianjin Health Committee (No. 2021099) and Tianjin Education Committee (No. 2021KJ146).
Author information
Authors and Affiliations
Contributions
All authors had full access to all data in the study and were responsible for the integrity of the data and the accuracy of the data analysis. SL and JX proposed writing ideas; writing—original draft, SL, LZ, and JX; writing—review and editing, SL, LZ, RF, YG, and YZ; visualization, SL and LZ; supervision, SX and LZ; and project management, SL and SX.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Ethical statements
N/A.
Informed consent
N/A.
Animal and research involving recombinant DNA studies
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Zhao, L., Xiao, J. et al. The gut microbiome: an important role in neurodegenerative diseases and their therapeutic advances. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04853-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04853-6