Abstract
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection leads to hyper-inflammation and amplified immune response in severe cases that may progress to cytokine storm and multi-organ injuries like acute respiratory distress syndrome and acute lung injury. In addition to pro-inflammatory cytokines, different mediators are involved in SARS-CoV-2 pathogenesis and infection, such as sphingosine-1-phosphate (S1P). S1P is a bioactive lipid found at a high level in plasma, and it is synthesized from sphingomyelin by the action of sphingosine kinase. It is involved in inflammation, immunity, angiogenesis, vascular permeability, and lymphocyte trafficking through G-protein coupled S1P receptors. Reduction of the circulating S1P level correlates with COVID-19 severity. S1P binding to sphingosine-1-phosphate receptor 1 (S1PR1) elicits endothelial protection and anti-inflammatory effects during SARS-CoV-2 infection, by limiting excessive INF-α response and hindering mitogen-activated protein kinase and nuclear factor kappa B action. However, binding to S1PR2 opposes the effect of S1PR1 with vascular inflammation, endothelial permeability, and dysfunction as the concomitant outcome. This binding also promotes nod-like receptor pyrin 3 (NLRP3) inflammasome activation, causing liver inflammation and fibrogenesis. Thus, higher expression of macrophage S1PR2 contributes to the activation of the NLRP3 inflammasome and the release of pro-inflammatory cytokines. In conclusion, S1PR1 agonists and S1PR2 antagonists might effectively manage COVID-19 and its severe effects. Further studies are recommended to elucidate the potential conflict in the effects of S1P in COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus virus disease 2019 (COVID-19) is the third global respiratory viral pandemic that was first reported in December 2019, following the Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV) in 2012 and 2003, respectively [1, 2]. COVID-19 is caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), a single RNA virus from the Betacoronaviridae family [3, 4]. This virus exploits different receptors for entry into the host cells, angiotensin-converting enzyme 2 (ACE2) is reported as the main entry receptor. SARS-CoV-2 infection is associated with the development of excessive immune response and hyper-inflammation in severe cases, which may progress to cytokine storm and multi-organ injuries (MOI) like acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) [5,6,7].
In addition to the pro-inflammatory cytokines, different mediators are involved in SARS-CoV-2 pathogenesis and infection, like sphingosine-1-phosphate (S1P) [8, 9]. S1P is a bioactive lipid found at a high level in plasma, and it is synthesized from sphingomyelin by the action of sphingosine kinase (Sphk1/2), ceramidase, and sphingomyelinase [10]. S1P is metabolized to an inactive form by S1P lyase (SPL) (Fig. 1).
Characteristics of sphingosine-1 phosphate
The main sources of S1P are erythrocytes, endothelial cells, and platelets to a lesser extent [10]. S1P activates G-protein coupled S1P receptors (SIPR) 1–5, with the endothelial cells highly expressing S1PR1-3. S1P is involved in inflammation, immunity, angiogenesis, vascular permeability, and lymphocyte trafficking [11]. Pharmacological inhibition of Sphk1/2 halves S1P plasma concentration. Interestingly, Sphk1/2 null mice surprisingly show a higher S1P level since Sphk1/2 participates in the redistribution of S1P between erythrocytes and endothelial cells [10].
S1PR1 is the most common and widely distributed of all the receptors for S1P. Dynamin-2 and clathrin mediate this receptor to activate the phosphatidylinositol 3-kinase (PI3K) pathway, which is essential for maintaining vascular permeability and stabilization [12]. S1PR1 improves innate immunity by activating macrophages and neutrophil migrations, mast and eosinophilic cells, and inhibiting abnormal interferon-alpha (IFN-α) production during viral infections [13]. S1PR1 regulates innate and adaptive immune responses by controlling natural killer (NK) cell trafficking and macrophage polarization [13]. These downstream signalings enhance endothelial protection and anti-inflammatory effects.
Emerging evidence from previous and recent studies demonstrates that S1P via S1PR1 causes vasodilation and endothelial protection through a nitric oxide (NO) dependent pathway and modulation of Ca + 2 transports [14, 15]. It has been illustrated experimentally that S1P-deficient mice subjected to anaphylaxis exhibit high vascular leakage and mortality. In this condition, the administration of erythrocytes, a main source of S1P, restored endothelial function and integrity in S1P-deficient mice [16]. Therefore, S1PR1 elicits barrier and cardioprotective properties through anti-inflammatory activity.
On the other hand, S1PR2 in response to S1P opposes the effect of S1PR1. It induces phagocytosis independent of complement activation by inhibiting S1PR1-mediated signaling pathways while inducing vascular permeability and endothelial dysfunction [17]. Thus, the expression balance between S1PR1 and S1PR2 may affect the endothelial response to S1P. A better consideration and understanding of how S1P produces beneficial or harmful effects on disease and health should be related to the receptor types.
S1P binding to S1PR2 also antagonizes S1PR1 action via activation of the G12/13-Rho-Rho kinase (ROCK) pathway, which induces endothelial permeability [17]. Endothelial dysfunction is developed during acute inflammation with the progression of adhesion molecules. S1PR2 is instrumental in vascular inflammation and inhibition of S1PR1, promoting the development of endothelial dysfunction, vascular inflammation, and ischemic-reperfusion injury [18].
Role of S1P pathway in viral infections
The SphK1/2/S1P axis has a potential role in the generation and release of pro-inflammatory cytokines and vascular integrity. S1P plays an integral role in regulating viral replication, adaptive/innate immune response, and hyperinflammation [19]. Activation of Sphk1/2 accelerates infections such as respiratory syncytial virus (RSV) and cytomegalovirus (CMV) infections, while its inhibition truncates viral replication of measles virus (MV) and influenza A virus [20]. The SphK1/S1P axis facilitates viral entry and improves viral replication. S1P acts as a co-receptor modulating viral entry, intracellular replication, and affects antiviral immune response [19]. It has been demonstrated that cells over-expressing SphK1 are highly susceptible to different viral infections compared to normal cells [21]. Recently, SphK1 has been shown to be co-localized with viral RNA, so inhibition of Sphk1/2 may impair and limit viral replication [21].
Different viruses, such as dengue virus, RSV, CMV, measles, hepatitis C virus (HCV), hepatitis B virus (HBV), and influenza virus use the SphK1/2/S1P axis in their replications [22]. Notably, influenza virus infection promotes the expression of SphK1/S1P with subsequent expression and activation of inflammatory signaling pathways [23]. Besides, MV provokes the expression of SphK1/2/S1P through inducing heat shock protein 90 (HS90) expression and mechanistic targeting of the rapamycin (mTOR) pathway, which are critical for viral replication [24].
These verdicts suggest that the SphK1/2/S1P axis has an important role in viral replication and that inhibitors of this intracellular axis may restrict viral load by inhibiting viral replication. Targeting the SphK1/2/S1P axis could be an effective strategy against viral infections and associated hyperinflammation and endothelial dysfunction.
Role of S1P pathway in COVID-19
The SphK1/2/S1P axis is also involved in promoting the replication of SARS-CoV-2 and the release of pro-inflammatory cytokines [22]. Pan et al. [25] suggest that the SphK1/2/S1P pathway promotes the invasion of SARS-CoV-2 into the central nervous system (CNS) through the olfactory pathway by expressing S1PR1. It has been observed that low levels of plasma S1P were correlated with COVID-19 severity and can be regarded as a biomarker of disease severity [26]. In their prospective case–control study involving 111 COVID-19 patients and 47 healthy controls, Marfia et al. found that the reduction in circulating S1P level is correlated with COVID-19 severity [27]. The underlying mechanisms were due to either injury to endothelial cells, erythrocytes, and platelets, which are major sources of circulating S1P, or reduction of S1P transporters like high-density lipoprotein (HDL) and albumin [27].
Moreover, S1P is increased within the erythrocytes by upregulation of Sphk1/2 in COVID-19 patients as an adaptive response to maintain endothelial integrity and prevent tissue hypoxia [28, 29]. However, hemolytic anemia and abnormal erythrocrine function in severe COVID-19 may affect the circulating S1P [30, 31]. S1P is rapidly synthesized from endothelial cells and hemopoietic cells to compensate any reduction in plasma S1P [11]. Though, in severe COVID-19 due to the suppression of hemopoietic tissues by high circulating IL-6, serum S1P is reduced and correlates with disease severity [27].
Despite these robust findings, these observations did not discuss the receptor-dependent effects of S1P and further insight into the resultant benefits or detriments. Naz and Arish, 2020 reported that S1P limits excessive IFN-α response in SARS-CoV-2 infection by down-streaming of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) [32]. Thus, activation of S1PR1 and inhibition of S1PR2 could be beneficial in COVID-19 sufferers, as S1P analogue(s) might be helpful in treating COVID-19.
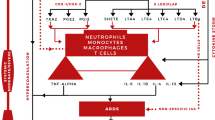
S1PR2 contributes to TNF-α-induced pro-inflammatory response and NF-κB activation, developing endothelial permeability and dysfunction [33,34,35]. Hou and colleagues revealed that S1P through S1PR2 promotes nod-like receptor pyrin 3 (NLRP3) inflammasome activation, causing liver inflammation and fibrogenesis [36]. Thus, higher expression of S1PR2 by macrophages contributes to the activation of the NLRP3 inflammasome and pro-inflammatory cytokine release. It has been shown that NLRP3 inflammasome and pro-inflammatory cytokines are highly activated in COVID-19 and are linked with the development of cytokine storm and ALI/ARDS [37,38,39]. Different studies revealed that the SphK-S1P-S1PR axis plays a role in accelerating inflammation and growth of endometriotic cells by increasing the expression of IL-6 and other pro-inflammatory cytokines [40]. As well, 1P has been shown to regulate cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) expression and IL-6 secretion in various respiratory diseases [41]. However, the mechanisms underlying S1P-induced COX-2 expression and PGE2 production in human tracheal smooth muscle cells (HTSMCs) remain unclear [42]. However, S1P-induced COX-2 expression and PGE2/IL-6 generation was mediated through S1PR2 [42].
S1P inhibits ALI via S1PR1, whereas S1PR2 causes ALI and pulmonary edema [43]. Zhu et al. found that ApoM produces a protective effect against ALI through S1P/S1PR1 [44]. These findings suggest receptor-dependent effects of S1P in inducing ALI in COVID-19. Moreover, S1PR2 is induced during hypoxia [45], a cardinal feature of patients with severe COVID-19 [46]. In their research, Michaud et al. observed that S1P is regarded as a novel non-hypoxic stimulus that induces hypoxia-inducible factor (HIF-1) [47]. It has been proposed that high HIF-1 may protect against COVID-19 severity by decreasing ferritin and modulating ACE2 expression [48, 49]. Hence, the protective role of S1P in COVID-19 is exerted through S1PR1 binding and responsive downstream effect.
S1P via activation of S1PR2 inhibits the egress of lymphocytes from lymphoid organs, which causes lymphopenia, a condition linked with COVID-19 severity [50, 51]. Fingolimod, a modulator of S1PR, can sequester lymphocytes in the lymph nodes, preventing them from contributing to the development of autoimmune disorders such as multiple sclerosis [52]. This medication is an analogue of sphingosine phosphorylated by Sphk to yield phospho-fingolimod. This intermediate, upon binding to SIPR1, induces the internalization of S1PR1 and sequestration of lymphocytes [53]. Various studies have also reported a rise in mice and humans' blood pressure following long-term treatment with fingolimod [54, 55]. Furthermore, higher expression of S1PR2 in the lung may lead to pulmonary vasoconstriction and the development of pulmonary hypertension [56], which are hallmarks of severe COVID-19 [57]. Besides, expression of S1PR2 causes disruption of endothelial integrity and the development of endothelial dysfunction by reducing endothelial nitric oxide synthase (eNOS) and NO availability, triggering the release of pro-inflammatory cytokines [58]. Bonaventura et al. observed that endothelial dysfunction is a potential cause of immunothrombosis and ALI/ARDS progression [59, 60]. It has been reported that both S1PR1 and S1PR2 promote platelet activation and thrombin formation [61]. On that account, S1P-induced endothelial dysfunction and coagulopathy could increase COVID-19 severity. However, glucocorticoid anti-inflammatory effects are partially mediated by the activation of Sphk1 and activation of the S1P/S1PR2 complex [62], making them effective in COVID-19 by inhibiting IL-6-induced S1P release [27].
During inflammation, tumor necrosis factor-alpha (TNF-α) activates Sphk1/2 in the endothelial cells, increasing S1P synthesis, and expression of S1PR2 [33]. This effect triggers the development of endothelial dysfunction and immunothrombosis in COVID-19 through an S1PR2-dependent pathway [33]. Targeting of Sphk1/2 by specific inhibitors may inhibit S1PR2-mediated hyperinflammation and endothelial dysfunction [22]. However, inhibiting Sphk1/2 may have a negative impact on SARS-CoV-2 infection because using the S1P analogue FTY720/fingolimod reduces hyperinflammation and limits immune response exaggeration during SARS-CoV-2 infection [63]. Similarly, the sphingolipid derivative, ceramaid-1 phosphate, exhibits immunoregulatory and antiviral effects by which it enhances antigen presentation and autophagy with activation of T cell response that may be beneficial in the case of SARS-CoV-2 infection [63].
Moreover, SARS-CoV-2-induced up-regulation of the renin-angiotensin system (RAS) promotes the progression and elevation of circulating angiotensin 2 (Ang-II), promoting the release of pro-inflammatory cytokines with the subsequent development of endothelial dysfunction, vascular inflammation, and ALI/ARDS [60, 64, 65]. It is worth noting that S1P via S1PR1 can cause cardiac remodeling and fibrosis by inducing the release of Ang-II and IL-6 [66]. Meissner and his colleagues, 2017 illustrated that S1P is a kingpin in the pathogenesis of Ang-II-induced hypertension [67]. These findings suggest that S1P could be a detrimental factor in increasing cardiovascular instability in COVID-19. Furthermore, S1P is involved in SARS-CoV-2 pathogenesis and infection through transmembrane protease serine 2 (TMPRSS2)/ACE2 axis induction. In addition, activation of protective ACE2 is associated with the expression of S1P and S1PR [63, 68] (Fig. 2).
Modulation of the S1P pathway
The intonation of S1P receptors through agonists and antagonists is a common clinical intervention to achieve clinical utility. A common example is FTY720, an S1PR1 agonist that elicits an immunosuppressive effect through the inhibition of lymphocyte recirculation [69]. FTY20-P, the phosphorylated derivative of FTY20, binds to S1PR1 and acts as a functional agonist. FTY20-P is more potent than S1P in inducing the degradation and internalization of S1PR1. It also possesses anti-angiogenic and immunosuppressive properties, making it relevant for different inflammatory and autoimmune disorders [70]. S1P1 agonists attenuate the expression and release of pro-inflammatory cytokines including IL-6 during pathogenic influenza virus infection [71].
Similarly, ponesimod is a potent and orally active S1PR1 agonist, effective against lymphocyte-mediated inflammation, and used to manage autoimmune diseases [72]. According to Burg et al. in experimental mice with immune complex-induced endothelial dysfunction. ApoM-Fc, an S1PR1 agonist, attenuates the activation of polymorphonuclear neutrophils-induced endothelial dysfunction, suggesting S1PR1 agonists limit neutrophil escape from capillaries and enhance endothelial cell barriers, concomitantly preventing immune-mediated vascular injury [73]. Another S1PR1 agonist of interest is CYM-5442. When used in combination with the antiviral oseltamivir, it greatly protects against H1N1-induced ALI through the inhibition of activated MAPK and NF-κB signaling pathways [74]. In this regard, S1PR1 agonists may be useful in treating COVID-19 by dampening the exaggerated immune response and endothelial dysfunction that are hallmarks of SARS-CoV-2 infection [34]. Likewise, the S1PR1 agonist fingolimod could be a potential agent against SARS-CoV-2 infection-induced ALI/ARDS by inhibiting pulmonary vascular endothelial dysfunction and inflammatory infiltrate [74].
On the other hand, blocking of inflammatory S1PR2 by selective antagonists may reduce complement activation, vascular permeability, endothelial dysfunction, TNF-α-induced pro-inflammatory response, and NF-κB activation [18, 33]. JTE-013 is the only S1PR2 antagonist with well-understood and recognized pharmacology, such as low potency and selectivity [75]. Recently, other S1PR2 antagonists such as CYM-5520 and CYM-5578 have been identified, but there is a dearth of information in terms of characterization and understanding of their biological mechanisms. S1PR2 antagonists could be of value in reducing pulmonary hypertension and lung fibrosis. They may also attenuate endothelial dysfunction and restore vascular endothelial barriers [76]. As a consequence of their anti-inflammatory and endothelial cell-protective effects, S1PR2 antagonists might be of great value in managing COVID-19.
These observations highlight that S1P has a dual role in different viral infections, including SARS-CoV-2. Despite the different implications for the role of the SphK1/2/S1P axis in the enhancement of viral infections, S1P exerts a protective role through an S1PR1-dependent pathway against the propagation of endothelial dysfunction and the release of pro-inflammatory cytokines. However, S1P via an S1PR2-dependent pathway provokes an inflammatory reaction and the induction of endothelial permeability. Therefore, S1PR1 agonists and S1PR2 antagonists could be a novel therapeutic strategy against SARS-CoV-2. In this sense, this brief review, unlike other studies that focused on the level of SIP in COVID-19, provides a new idea regarding the receptor-dependent effect of SIP. S1PR1 agonists and S1PR2 antagonists may offer a novel approach to COVID-19 management by modulating the exaggerated immuno-inflammatory response against SARS-CoV-2 infection, as well as the associated endothelial dysfunction and triggered inflammatory signaling pathways (Fig 3).
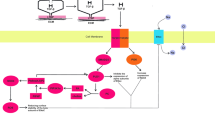
Role of S1P receptors in COVID-19. S1P via the activation of S1P receptor 1 (S1PR1), activates phosphoinositol 3 kinase (PI3K), which maintains vascular permeability and inhibits the development of endothelial dysfunction (ED). The activation of S1PR1 stimulates interferon alpha (INF-α) which inhibits viral infection and decreases viral load. This activation inhibits the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). The activation of S1PR2 induces the release of pro-inflammatory cytokines (PIC) and the development of hyperinflammation. S1PR2 also triggers vascular permeability with the development of ED. Thus, the activation of S1PR2 increases the risk of development of ALI/ARDS
Conclusion
This review demonstrates the S1P’s potential role in COVID-19 with reference to its receptor-dependent effects. These observations give a controversial picture of the potential role of S1P in COVID-19 due to poor evaluation of receptor-specific effects. In contrast to the adverse consequences of the S1P-S1PR2 binding, which include endothelial dysfunction and the production of coagulopathy, the S1P-S1PR1 binding has protective effects. Therefore, S1PR1 agonists and S1PR2 antagonists, regardless of S1P level, might be a novel therapeutic approach for managing COVID-19 and its severe effects. Further studies are recommended to find agents with dual S1PR1 agonists /S1PR2 antagonists’ activity and elucidate their effects on COVID-19. Elucidating the potential conflict in the effects of S1P in COVID-19 is highly recommended.
Data availability
All data considered during this review is presented within the manuscript.
References
Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE (2021) The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem 476(11):4161–4166. https://doi.org/10.1007/s11010-021-04232-z
Babalghith AO, Al-Kuraishy HM, Al-Gareeb AI, De Waard M, Al-Hamash SM, Jean-Marc S, Negm WA, Batiha GE (2022) The role of berberine in Covid-19: potential adjunct therapy. Inflammopharmacology 30(6):2003–2016. https://doi.org/10.1007/s10787-022-01080-1
Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE (2021) Niclosamide for Covid-19: bridging the gap. Mol Biol Rep 48(12):8195–8202. https://doi.org/10.1007/s11033-021-06770-7
Al-Kuraishy HM, Batiha GE, Faidah H, Al-Gareeb AI, Saad HM, Simal-Gandara J (2022) Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology 30(6):2017–2026. https://doi.org/10.1007/s10787-022-01027-6
Iheagwam FN, Rotimi SO (2020) Computer-aided analysis of multiple SARS-CoV-2 therapeutic targets: identification of potent molecules from African medicinal plants. Scientifica 2020:1878410. https://doi.org/10.1155/2020/1878410
Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE (2021) Covid-19 and development of heart failure: mystery and truth. Naunyn Schmiedebergs Arch Pharmacol 394(10):2013–2021. https://doi.org/10.1007/s00210-021-02147-6
Batiha GE, Al-Gareeb AI, Elekhnawy E, Al-Kuraishy HM (2022) Potential role of lipoxin in the management of COVID-19: a narrative review. Inflammopharmacology 30(6):1993–2001. https://doi.org/10.1007/s10787-022-01070-3
Batiha GE, Al-Kuraishy HM, Al-Gareeb AI, Alruwaili M, AlRuwaili R, Albogami SM, Alorabi M, Saad HM, Simal-Gandara J (2022) Targeting of neuroinflammation by glibenclamide in Covid-19: old weapon from arsenal. Inflammopharmacology 23:1–7. https://doi.org/10.1007/s10787-022-01087-8
Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GE (2022) Nitazoxanide and COVID-19: a review. Mol Biol Rep 49(11):11169–11176. https://doi.org/10.1007/s11033-022-07822-2
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22(1):50–60. https://doi.org/10.1016/j.tcb.2011.09.003
Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T (2008) Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102(6):669–676. https://doi.org/10.1161/CIRCRESAHA.107.165845
Pyne NJ, Pyne S (2017) Sphingosine 1-phosphate receptor 1 signaling in mammalian cells. Molecules (Basel, Switzerland) 22(3):344. https://doi.org/10.3390/molecules22030344
Bryan AM, Del Poeta M (2018) Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol 20(5):e12836. https://doi.org/10.1111/cmi.12836
Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ (2007) Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 292(1):R440–R446. https://doi.org/10.1152/ajpregu.00085.2006
Jozefczuk E, Guzik TJ, Siedlinski M (2020) Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol Res 156:104793. https://doi.org/10.1016/j.phrs.2020.104793
Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR (2009) Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Investig 119(7):1871–1879. https://doi.org/10.1172/jci38575
Okamoto Y, Kitakaze K, Takenouchi Y, Yamamoto S, Ishimaru H, Tsuboi K (2021) Sphingosine 1-phosphate receptor type 2 positively regulates interleukin (IL)-4/IL-13-induced STAT6 phosphorylation. Cell Signal 88:110156. https://doi.org/10.1016/j.cellsig.2021.110156
Hait NC, Maiti A (2017) The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat Inflamm 2017:4806541. https://doi.org/10.1155/2017/4806541
Carr JM, Mahalingam S, Bonder CS, Pitson SM (2013) Sphingosine kinase 1 in viral infections. Rev Med Virol 23(2):73–84. https://doi.org/10.1002/rmv.1718
Meacci E, Garcia-Gil M, Pierucci F (2020) SARS-CoV-2 infection: a role for S1P/S1P receptor signaling in the nervous system? Int J Mol Sci 21(18):6773. https://doi.org/10.3390/ijms21186773
Wolf JJ, Studstill CJ, Hahm B (2019) Emerging connections of S1P-metabolizing enzymes with host defense and immunity during virus infections. Viruses 11(12):1097. https://doi.org/10.3390/v11121097
McGowan EM, Haddadi N, Nassif NT, Lin Y (2020) Targeting the SphK-S1P-SIPR pathway as a potential therapeutic approach for COVID-19. Int J Mol Sci 21(19):7189. https://doi.org/10.3390/ijms21197189
Oldstone MBA, Rosen H (2014) Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. In: Oldstone M, Rosen H (eds) Sphingosine-1-phosphate signaling in immunology and infectious diseases. Current topics in microbiology and immunology, vol 378. Springer, Cham. https://doi.org/10.1007/978-3-319-05879-5_6
Grafen A, Schumacher F, Chithelen J, Kleuser B, Beyersdorf N, Schneider-Schaulies J (2019) Use of acid ceramidase and sphingosine kinase inhibitors as antiviral compounds against measles virus infection of lymphocytes in vitro. Front Cell Dev Biol 7:218. https://doi.org/10.3389/fcell.2019.00218
Pan Y, Gao F, Zhao S, Han J, Chen F (2021) Role of the SphK-S1P-S1PRs pathway in invasion of the nervous system by SARS-CoV-2 infection. Clin Exp Pharmacol Physiol 48(5):637–650. https://doi.org/10.1111/1440-1681.13483
Törnquist K, Asghar MY, Srinivasan V, Korhonen L, Lindholm D (2021) Sphingolipids as modulators of SARS-CoV-2 infection. Front Cell Dev Biol 9:689854. https://doi.org/10.3389/fcell.2021.689854
Marfia G, Navone S, Guarnaccia L, Campanella R, Mondoni M, Locatelli M, Barassi A, Fontana L, Palumbo F, Garzia E, Ciniglio Appiani G, Chiumello D, Miozzo M, Centanni S, Riboni L (2021) Decreased serum level of sphingosine-1-phosphate: a novel predictor of clinical severity in COVID-19. EMBO Mol Med 13(1):e13424. https://doi.org/10.15252/emmm.202013424
Winkler MS, Claus RA, Schilder M, Pöhlmann S, Coldewey SM, Grundmann J, Fricke T, Moerer O, Meissner K, Bauer M, Hofmann-Winkler H, Gräler MH (2021) Erythrocytes increase endogenous sphingosine 1-phosphate levels as an adaptive response to SARS-CoV-2 infection. Clin Sci (London, England : 1979) 135(24):2781–2791. https://doi.org/10.1042/CS20210666
Alkhayyat SS, Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, AboKamer AM, Batiha GE, Simal-Gandara J (2022) Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: the missed key for Holy Grail. Inflamm Res 71(10–11):1159–1167. https://doi.org/10.1007/s00011-022-01615-w
Al-Kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE (2022) Hemolytic anemia in COVID-19. Ann Hematol 101(9):1887–1895. https://doi.org/10.1007/s00277-022-04907-7
Al-Kuraishy HM, Al-Gareeb AI, Onohuean H, El-Saber Batiha G (2022) COVID-19 and erythrocrine function: the roller coaster and danger. Int J Immunopathol Pharmacol 36:3946320221103151. https://doi.org/10.1177/03946320221103151
Naz F, Arish M (2020) Battling COVID-19 pandemic: sphingosine-1-phosphate analogs as an adjunctive therapy? Front Immunol 11:1102. https://doi.org/10.3389/fimmu.2020.01102
Bernacchioni C, Ghini V, Squecco R, Idrizaj E, Garella R, Puliti E, Cencetti F, Bruni P, Donati C (2021) Role of sphingosine 1-phosphate signalling axis in muscle atrophy induced by TNFα in C2C12 myotubes. Int J Mol Sci 22(3):1280. https://doi.org/10.3390/ijms22031280
Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE (2022) Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int Immunopharmacol 104:108516. https://doi.org/10.1016/j.intimp.2021.108516
Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE (2022) High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology 30(3):811–820. https://doi.org/10.1007/s10787-022-00988-y
Hou L, Yang L, Chang N, Zhao X, Zhou X, Dong C, Liu F, Yang L, Li L (2020) Macrophage sphingosine 1-phosphate receptor 2 blockade attenuates liver inflammation and fibrogenesis triggered by NLRP3 inflammasome. Front Immunol 11:1149. https://doi.org/10.3389/fimmu.2020.01149
Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, El-Saber Batiha G (2021) Pleiotropic effects of tetracyclines in the management of Covid-19: emerging perspectives. Front Pharmacol 12:642822. https://doi.org/10.3389/fphar.2021.642822
Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ, Alexiou A, Mukerjee N, Batiha GE (2022) An insight into the placental growth factor (PlGf)/angii axis in Covid-19: a detrimental intersection. Biotechnol Genet Eng Rev. https://doi.org/10.1080/02648725.2022.2122291
Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ, Alexiou A, Mukerjee N, Batiha GE (2022) Prostaglandins and non-steroidal anti-inflammatory drugs in Covid-19. Biotechnol Genet Eng Rev. https://doi.org/10.1080/02648725.2022.2122290
Yoshino O, Yamada-Nomoto K, Kano K, Ono Y, Kobayashi M, Ito M, Yoneda S, Nakashima A, Shima T, Onda T, Osuga Y, Aoki J, Saito S (2019) Sphingosine 1 phosphate (S1P) increased IL-6 expression and cell growth in endometriotic cells. Reprod Sci (Thousand Oaks, Calif.) 26(11):1460–1467. https://doi.org/10.1177/1933719119828112
Nadwa EH, Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E, Albogami SM, Alorabi M, Batiha GE, De Waard M (2022) Cholinergic dysfunction in COVID-19: frantic search and hoping for the best. Naunyn-Schmiedeberg’s Arch Pharmacol. https://doi.org/10.1007/s00210-022-02346-9
Hsu CK, Lee IT, Lin CC, Hsiao LD, Yang CM (2015) Sphingosine-1-phosphate mediates COX-2 expression and PGE2 /IL-6 secretion via c-Src-dependent AP-1 activation. J Cell Physiol 230(3):702–715. https://doi.org/10.1002/jcp.24795
Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, Mathew B, Zhao Y, Wang L, Bittman R, Weichselbaum R, Berdyshev E, Garcia JG (2013) Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol 49(1):6–17. https://doi.org/10.1165/rcmb.2012-0411TR
Zhu B, Luo GH, Feng YH, Yu MM, Zhang J, Wei J, Yang C, Xu N, Zhang XY (2018) Apolipoprotein M protects against lipopolysaccharide-induced acute lung injury via sphingosine-1-phosphate signaling. Inflammation 41(2):643–653. https://doi.org/10.1007/s10753-017-0719-x
Nicholson CK, Lambert JP, Molkentin JD, Sadoshima J, Calvert JW (2013) Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler Thromb Vasc Biol 33(4):744–751. https://doi.org/10.1161/ATVBAHA.112.300484
Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE (2021) COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with Type II diabetes mellitus: the anti-inflammatory role of metformin. Front Med 8:644295. https://doi.org/10.3389/fmed.2021.644295
Michaud MD, Robitaille GA, Gratton JP, Richard DE (2009) Sphingosine-1-phosphate: a novel nonhypoxic activator of hypoxia-inducible factor-1 in vascular cells. Arterioscler Thromb Vasc Biol 29(6):902–908. https://doi.org/10.1161/ATVBAHA.109.185280
Afsar B, Kanbay M, Afsar RE (2020) Hypoxia inducible factor-1 protects against COVID-19: a hypothesis. Med Hypotheses 143:109857. https://doi.org/10.1016/j.mehy.2020.109857
Al-kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GE (2022) Dipyridamole and adenosinergic pathway in Covid-19: a juice or holy grail. Egypt J Med Hum Genet 23:140. https://doi.org/10.1186/s43042-022-00354-1
Moriyama S, Takahashi N, Green JA, Hori S, Kubo M, Cyster JG, Okada T (2014) Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J Exp Med 211(7):1297–1305. https://doi.org/10.1084/jem.20131666
Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M (2021) Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand J Immunol 93(2):e12967. https://doi.org/10.1111/sji.12967
Sanford M (2014) Fingolimod: a review of its use in relapsing-remitting multiple sclerosis. Drugs 74(12):1411–1433. https://doi.org/10.1007/s40265-014-0264-y
Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ (2001) Lysophospholipids–receptor revelations. Science (New York, N.Y.) 294(5548):1875–1878. https://doi.org/10.1126/science.1065323
Cantalupo A, Di Lorenzo A (2016) S1P signaling and de novo biosynthesis in blood pressure homeostasis. J Pharmacol Exp Ther 358(2):359–370. https://doi.org/10.1124/jpet.116.233205
Li K, Konofalska U, Akgün K, Reimann M, Rüdiger H, Haase R, Ziemssen T (2017) Modulation of cardiac autonomic function by fingolimod initiation and predictors for fingolimod induced bradycardia in patients with multiple sclerosis. Front Neurosci 11:540. https://doi.org/10.3389/fnins.2017.00540
Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF (2011) S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circul 1(3):399–404. https://doi.org/10.4103/2045-8932.87309
Feng WX, Yang Y, Wen J, Liu YX, Liu L, Feng C (2021) Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension. ESC Heart Fail 8(1):714–718. https://doi.org/10.1002/ehf2.13023
Liao J, Zheng Y, Hu M, Xu P, Lin L, Liu X, Wu Y, Huang B, Ye X, Li S, Duan R, Fu H, Huang J, Wen L, Fu Y, Kilby MD, Kenny LC, Baker PN, Qi H, Tong C (2022) Impaired sphingosine-1-phosphate synthesis induces preeclampsia by deactivating trophoblastic YAP (yes-associated protein) through S1PR2 (sphingosine-1-phosphate receptor-2)-induced actin polymerizations. Hypertension (Dallas, Tex.: 1979) 79(2):399–412. https://doi.org/10.1161/HYPERTENSIONAHA.121.18363
Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A (2021) Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 21(5):319–329. https://doi.org/10.1038/s41577-021-00536-9
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Alexiou A, Batiha GE (2022) Calprotectin: the link between acute lung injury and gastrointestinal injury in covid-19: ban or boon. Curr Protein Pept Sci 23(5):310–320. https://doi.org/10.2174/1389203723666220610124303
Liu H, Jackson ML, Goudswaard LJ, Moore SF, Hutchinson JL, Hers I (2021) Sphingosine-1-phosphate modulates PAR1-mediated human platelet activation in a concentration-dependent biphasic manner. Sci Rep 11(1):15308. https://doi.org/10.1038/s41598-021-94052-4
Vettorazzi S, Bode C, Dejager L, Frappart L, Shelest E, Klaßen C, Tasdogan A, Reichardt HM, Libert C, Schneider M, Weih F, Henriette Uhlenhaut N, David JP, Gräler M, Kleiman A, Tuckermann JP (2015) Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun 6:7796. https://doi.org/10.1038/ncomms8796
Prakash H, Upadhyay D, Bandapalli OR, Jain A, Kleuser B (2021) Host sphingolipids: perspective immune adjuvant for controlling SARS-CoV-2 infection for managing COVID-19 disease. Prostaglandins Other Lipid Mediat 152:106504. https://doi.org/10.1016/j.prostaglandins.2020.106504
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Al-Buhadily AK, Al-Harchan NA, Lugnier C (2020) COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J Microsc Ultrastruct 8(4):141–145. https://doi.org/10.4103/JMAU.JMAU_63_20
Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE (2022) Central effects of ivermectin in alleviation of Covid-19-induced Dysauto-nomia. Curr Drug Targets 23(13):1277–1287. https://doi.org/10.2174/1389450123666220810102406
Ohkura SI, Usui S, Takashima SI, Takuwa N, Yoshioka K, Okamoto Y, Inagaki Y, Sugimoto N, Kitano T, Takamura M, Wada T, Kaneko S, Takuwa Y (2017) Augmented sphingosine 1 phosphate receptor-1 signaling in cardiac fibroblasts induces cardiac hypertrophy and fibrosis through angiotensin II and interleukin-6. PLoS ONE 12(8):e0182329. https://doi.org/10.1371/journal.pone.0182329
Meissner A, Miro F, Jiménez-Altayó F, Jurado A, Vila E, Planas AM (2017) Sphingosine-1-phosphate signalling-a key player in the pathogenesis of Angiotensin II-induced hypertension. Cardiovasc Res 113(2):123–133. https://doi.org/10.1093/cvr/cvw256
Al-Kuraishy HM, Al-Buhadily AK, Al-Gareeb AI, Alorabi M, Hadi Al-Harcan NA, El-Bouseary MM, Batiha GE (2022) Citicoline and COVID-19: vis-à-vis conjectured. Naunyn Schmiedebergs Arch Pharmacol 395(12):1463–1475. https://doi.org/10.1007/s00210-022-02284-6
Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science (New York, N.Y.) 296(5566):346–349. https://doi.org/10.1126/science.1070238
Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T (2007) Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem 282(12):9082–9089. https://doi.org/10.1074/jbc.M610318200
Zhao J, Zhu M, Jiang H, Shen S, Su X, Shi Y (2019) Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus. Sci Rep 9(1):5272. https://doi.org/10.1038/s41598-019-41760-7
Piali L, Froidevaux S, Hess P, Nayler O, Bolli MH, Schlosser E, Kohl C, Steiner B, Clozel M (2011) The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther 337(2):547–556. https://doi.org/10.1124/jpet.110.176487
Burg N, Swendeman S, Worgall S, Hla T, Salmon JE (2018) Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthr Rheumatol (Hoboken, N.J.) 70(11):1879–1889. https://doi.org/10.1002/art.40558
Vahed SZ, Ghiyasvand S, Khatibi SMH, Patel B, Shoja MM, Tolouian R et al (2021) Sphingosine 1 phosphate agonists (SPI); a potential agent to prevent acute lung injury in COVID-19. Immunopathol Persa 7(1):e03. https://doi.org/10.34172/ipp.2021.03
Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y (2002) Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299(3):483–487. https://doi.org/10.1016/s0006-291x(02)02671-2
Blankenbach KV, Schwalm S, Pfeilschifter J, Heringdorf DMZ (2016) Sphingosine-1-phosphate receptor-2 antagonists: therapeutic potential and potential risks. Front Pharmacol 7:167. https://doi.org/10.3389/fphar.2016.00167
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HMA and GEB wrote the first draft of the manuscript. HMA, AIA, NAH, and NNW designed the figures and checked and revised the draft manuscript. All authors contributed, read, revised, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-kuraishy, H.M., Batiha, G.ES., Al-Gareeb, A.I. et al. Receptor-dependent effects of sphingosine-1-phosphate (S1P) in COVID-19: the black side of the moon. Mol Cell Biochem 478, 2271–2279 (2023). https://doi.org/10.1007/s11010-023-04658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04658-7