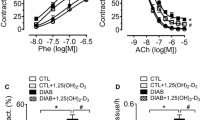
Abstract
Monoamine oxidases (MAOs), mitochondrial enzymes with two isoforms, A and B, have been recently recognized as significant contributors to oxidative stress in the cardiovascular system. The present study was purported to assess the effect of metformin and empagliflozin on MAO expression, oxidative stress and vascular reactivity in internal mammary arteries harvested from overweight patients with coronary heart disease subjected to bypass grafting. Vascular rings were prepared and acutely incubated (12 h) with high glucose (GLUC, 400 mg/dL) or angiotensin II (AII, 100 nM) and metformin (10 µM) and/or empagliflozin (10 µM) and used for the assessment of MAO expression (qRT-PCR and immune histochemistry), reactive oxygen species (ROS, confocal microscopy and spectrophotometry), and vasomotor function (myograph). Ex vivo stimulation with GLUC or AII increased both MAOs expression, ROS production and impaired relaxation to acetylcholine (ACh) of the vascular rings. All effects were alleviated by incubation with each antidiabetic drug; no cumulative effect was obtained when the drugs were applied together. In conclusion, MAO-A and B are upregulated in mammary arteries after acute stimulation with GLUC and AII. Endothelial dysfunction and oxidative stress were alleviated by either metformin or empagliflozin in both stimulated and non-stimulated vascular samples harvested from overweight cardiac patients.



Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Yun JS, Ko SH (2021) Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 123:154838. https://doi.org/10.1016/j.metabol.2021.154838
Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB (2022) Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol 21:74. https://doi.org/10.1186/s12933-022-01516-6
Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, Di Palo KE, Golden SH, Sperling LS (2022) Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 145:e722–e759. https://doi.org/10.1161/cir.0000000000001040
Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, Lengyel C, Rhee NA, Russo GT, Shirabe S, Urbancova K, Vencio S (2021) Capture: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol 20:154. https://doi.org/10.1186/s12933-021-01344-0
Halabi A, Sen J, Huynh Q, Marwick TH (2020) Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol 19:124. https://doi.org/10.1186/s12933-020-01100-w
Hostalek U, Campbell I (2021) Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin 37:1705–1717. https://doi.org/10.1080/03007995.2021.1955667
Feng J, Wang X, Ye X, Ares I, Lopez-Torres B, Martínez M, Martínez-Larrañaga MR, Wang X, Anadón A, Martínez MA (2022) Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res 177:106114. https://doi.org/10.1016/j.phrs.2022.106114
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A (2021) 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. https://doi.org/10.1056/NEJMoa2107038
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation 145:e895–e1032. https://doi.org/10.1161/cir.0000000000001063
Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJS, Filippatos G, Greene SJ, McDonagh TA, Ponikowski P, Rosano G, Seferovic P, Vaduganathan M, Voors AA, Metra M (2022) Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 24:431–441. https://doi.org/10.1002/ejhf.2397
Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A (2020) Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol 27:494–510. https://doi.org/10.1177/2047487319870344
Antonucci S, Di Lisa F, Kaludercic N (2021) Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium 94:102344. https://doi.org/10.1016/j.ceca.2020.102344
Edmondson DE (2014) Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr Pharm Des 20:155–160. https://doi.org/10.2174/13816128113190990406
Heger J, Hirschhäuser C, Bornbaum J, Sydykov A, Dempfle A, Schneider A, Braun T, Schlüter KD, Schulz R (2021) Cardiomyocytes-specific deletion of monoamine oxidase B reduces irreversible myocardial ischemia/reperfusion injury. Free Radic Biol Med 165:14–23. https://doi.org/10.1016/j.freeradbiomed.2021.01.020
Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N (2010) Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106:193–202. https://doi.org/10.1161/circresaha.109.198366
Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, Paolocci N (2014) Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20:267–280. https://doi.org/10.1089/ars.2012.4616
Sturza A, Leisegang MS, Babelova A, Schroder K, Benkhoff S, Loot AE, Fleming I, Schulz R, Muntean DM, Brandes RP (2013) Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 62:140–146. https://doi.org/10.1161/hypertensionaha.113.01314
Sturza A, Duicu OM, Vaduva A, Danila MD, Noveanu L, Varro A, Muntean DM (2015) Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can J Physiol Pharmacol 93:555–561. https://doi.org/10.1139/cjpp-2014-0544
Lighezan R, Sturza A, Duicu OM, Ceausu RA, Vaduva A, Gaspar M, Feier H, Vaida M, Ivan V, Lighezan D, Muntean DM, Mornos C (2016) Monoamine oxidase inhibition improves vascular function in mammary arteries from nondiabetic and diabetic patients with coronary heart disease. Can J Physiol Pharmacol 94:1040–1047. https://doi.org/10.1139/cjpp-2015-0580
Sturza A, Olariu S, Ionică M, Duicu OM, Văduva AO, Boia E, Muntean DM, Popoiu CM (2019) Monoamine oxidase is a source of oxidative stress in obese patients with chronic inflammation (1). Can J Physiol Pharmacol 97:844–849. https://doi.org/10.1139/cjpp-2019-0028
Di Matteo S, Nevi L, Overi D, Landolina N, Faccioli J, Giulitti F, Napoletano C, Oddi A, Marziani AM, Costantini D, De Rose AM, Melandro F, Bragazzi MC, Grazi GL, Berloco PB, Giuliante F, Donato G, Moretta L, Carpino G, Cardinale V, Gaudio E, Alvaro D (2021) Metformin exerts anti-cancerogenic effects and reverses epithelial-to-mesenchymal transition trait in primary human intrahepatic cholangiocarcinoma cells. Sci Rep 11:2557. https://doi.org/10.1038/s41598-021-81172-0
Ionică LN, Gaiță L, Bînă AM, Soșdean R, Lighezan R, Sima A, Malița D, Crețu OM, Burlacu O, Muntean DM, Sturza A (2021) Metformin alleviates monoamine oxidase-related vascular oxidative stress and endothelial dysfunction in rats with diet-induced obesity. Mol Cell Biochem 476:4019–4029. https://doi.org/10.1007/s11010-021-04194-2
Merce AP, Ionică LN, Bînă AM, Popescu S, Lighezan R, Petrescu L, Borza C, Sturza A, Muntean DM, Creţu OM (2022) Monoamine oxidase is a source of cardiac oxidative stress in obese rats: the beneficial role of metformin. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04490-5
Alshnbari AS, Millar SA, O’Sullivan SE, Idris I (2020) Effect of sodium-glucose cotransporter-2 inhibitors on endothelial function: a systematic review of preclinical studies. Diabetes Ther 11:1947–1963. https://doi.org/10.1007/s13300-020-00885-z
Badalica-Petrescu M, Munteanu M, Sturza A, Noveanu L, Streian C, Socaciu C, Muntean D, Timar R, Dragan S (2014) Characterization of the effects of two polyphenols-rich plant extracts on isolated diabetic human mammary arteries. Rev Chim 65:861–864
Sun XQ, Peters EL, Schalij I, Axelsen JB, Andersen S, Kurakula K, Gomez-Puerto MC, Szulcek R, Pan X, da Silva GoncalvesBos D, Schiepers REJ, Andersen A, Goumans MJ, VonkNoordegraaf A, van der Laarse WJ, de Man FS, Bogaard HJ (2021) Increased MAO-A activity promotes progression of pulmonary arterial hypertension. Am J Respir Cell Mol Biol 64(3):331–343. https://doi.org/10.1165/rcmb.2020-0105OC
Sturza A, Popoiu CM, Ionică M, Duicu OM, Olariu S, Muntean DM, Boia ES (2019) Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev 2019:8954201. https://doi.org/10.1155/2019/8954201
Merce A, Buriman DG, Lascu A, Bînă AM, Feier HB, Petrescu L, Borza C, Sturza A, Muntean DM and Crețu OM (2022) Metformin reverses the effects of angiotensin 2 in human mammary arteries by modulating the expression of nitric oxide synthases. Serbian J Exp Clin Res. https://doi.org/10.2478/sjecr-2022-0070
Sambe T, Mason RP, Dawoud H, Bhatt DL, Malinski T (2018) Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomed Pharmacother 98:149–156. https://doi.org/10.1016/j.biopha.2017.12.023
Ding Y, Zhou Y, Ling P, Feng X, Luo S, Zheng X, Little PJ, Xu S, Weng J (2021) Metformin in cardiovascular diabetology: a focused review of its impact on endothelial function. Theranostics 11:9376–9396. https://doi.org/10.7150/thno.64706
Venu VKP, Saifeddine M, Mihara K, Faiza M, Gorobets E, Flewelling AJ, Derksen DJ, Hirota SA, Marei I, Al-Majid D, Motahhary M, Ding H, Triggle CR, Hollenberg MD (2021) Metformin prevents hyperglycemia-associated, oxidative stress-induced vascular endothelial dysfunction: essential role for the orphan nuclear receptor human nuclear receptor 4A1 (Nur77). Mol Pharmacol 100:428–455. https://doi.org/10.1124/molpharm.120.000148
Mohammed I, Hollenberg MD, Ding H, Triggle CR (2021) A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front Endocrinol (Lausanne) 12:718942. https://doi.org/10.3389/fendo.2021.718942
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP (2021) Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 6:148–158. https://doi.org/10.1001/jamacardio.2020.4511
Durante W, Behnammanesh G, Peyton KJ (2021) Effects of sodium-glucose co-transporter 2 inhibitors on vascular cell function and arterial remodeling. Int J Mol Sci. https://doi.org/10.3390/ijms22168786
Uthman L, Homayr A, Juni RP, Spin EL, Kerindongo R, Boomsma M, Hollmann MW, Preckel B, Koolwijk P, van Hinsbergh VWM, Zuurbier CJ, Albrecht M, Weber NC (2019) Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell Physiol Biochem 53:865–886. https://doi.org/10.33594/000000178
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S (2017) The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia 60:364–376. https://doi.org/10.1007/s00125-016-4158-2
Fukuda T, Bouchi R, Terashima M, Sasahara Y, Asakawa M, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y (2017) Ipragliflozin reduces epicardial fat accumulation in non-obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther 8:851–861. https://doi.org/10.1007/s13300-017-0279-y
Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M (2018) The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 17:6. https://doi.org/10.1186/s12933-017-0658-8
Park SH, Belcastro E, Hasan H, Matsushita K, Marchandot B, Abbas M, Toti F, Auger C, Jesel L, Ohlmann P, Morel O, Schini-Kerth VB (2021) Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol 20:65. https://doi.org/10.1186/s12933-021-01252-3
Campeau MA, Leask RL (2022) Empagliflozin mitigates endothelial inflammation and attenuates endoplasmic reticulum stress signaling caused by sustained glycocalyx disruption. Sci Rep 12:12681. https://doi.org/10.1038/s41598-022-16763-6
Uthman L, Li X, Baartscheer A, Schumacher CA, Baumgart P, Hermanides J, Preckel B, Hollmann MW, Coronel R, Zuurbier CJ, Weber NC (2022) Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na(+)](c)/ROS-pathway in human endothelial cells. Biomed Pharmacother 146:112515. https://doi.org/10.1016/j.biopha.2021.112515
Funding
This work was supported by the university internal funds awarded to the research centres.
Author information
Authors and Affiliations
Contributions
AL: original draft preparation, formal analysis; LNI: investigation, formal analysis; DGB: investigation, methodology; APM: investigation, methodology; LD: visualization; CB: visualization, supervision; OMC: project administration; AS: conceptualization, data curation, writing-review & editing; DMM: conceptualization, writing-review & editing, HBF: visualization, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Committee for Research Ethics of „Victor Babeș” University of Medicine and Pharmacy from Timișoara, Romania (No. 04/28.02.2020 and 04p/17/12/2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lascu, A., Ionică, L.N., Buriman, D.G. et al. Metformin and empagliflozin modulate monoamine oxidase-related oxidative stress and improve vascular function in human mammary arteries. Mol Cell Biochem 478, 1939–1947 (2023). https://doi.org/10.1007/s11010-022-04633-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04633-8