Abstract
Endocrine and paracrine fibroblast growth factor 23 (FGF23) is a protein predominantly produced by bone cells with strong impact on phosphate and vitamin D metabolism by targeting the kidney. Plasma FGF23 concentration early rises in kidney and cardiovascular diseases correlating with progression and outcome. Lactic acid is generated in anaerobic glycolysis. Lactic acidosis is the consequence of various physiological and pathological conditions and may be fatal. Since FGF23 production is stimulated by inflammation and lactic acid induces pro-inflammatory signaling, we investigated whether and how lactic acid influences FGF23. Experiments were performed in UMR106 osteoblast-like cells, Fgf23 mRNA levels estimated from quantitative real-time polymerase chain reaction, and FGF23 protein determined by enzyme-linked immunosorbent assay. Lactic acid dose-dependently induced Fgf23 gene expression and up-regulated FGF23 synthesis. Also, Na+-lactate as well as formic acid and acetic acid up-regulated Fgf23. The lactic acid effect was significantly attenuated by nuclear factor kappa-light-chain enhancer of activated B-cells (NFκB) inhibitors wogonin and withaferin A. Lactic acid induces FGF23 production, an effect at least in part mediated by NFκB. Lactic acidosis may, therefore, be paralleled by a surge in plasma FGF23.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone cells are the main source of fibroblast growth factor 23 (FGF23), a proteohormone with additional paracrine effects [1,2,3,4]. As an endocrine factor, it regulates vitamin D and phosphate homeostasis in the kidney by down-regulating CYP27B1, the key enzyme for activation of vitamin D, and NaPiIIa, the major Na+-dependent phosphate transporter [5,6,7,8]. In doing so, FGF23 inhibits the synthesis of 1,25(OH)2D3, active vitamin D [9], and enhances renal phosphate excretion resulting in lower plasma phosphate levels [10]. In the parathyroid glands, FGF23 decreases the secretion of parathyroid hormone (PTH) [11, 12]. Taken together, FGF23, 1,25(OH)2D3, and PTH are part of a complex hormone circuit influencing each other and controlling phosphate as well as Ca2+ homeostasis [5].
The aforementioned endocrine effects of FGF23 are dependent on a membrane receptor which assembles with transmembrane protein αKlotho [13,14,15]. Apart from being the co-receptor for FGF23, αKlotho has become known as a powerful anti-aging factor: Transmembrane αKlotho can release a fragment called soluble Klotho (sKL) with additional endocrine effects including anti-cancer activity [16,17,18,19]. FGF23 or αKlotho deficiency results in rapid aging and early onset of aging-associated diseases [14] whereas overexpression of αKlotho extends the life span of mice by about 30% [20].
Paracrine effects of FGF23 may affect the liver [21], heart [3, 22, 23], or immune system [24] and are, at least in part, αKlotho independent.
In clinical medicine, the plasma FGF23 concentration has been revealed as a valuable disease biomarker [25] which is positively correlated with progression and outcome in chronic kidney disease [26, 27] and further cardiovascular disorders [28,29,30].
Therefore, the regulation of FGF23 production is of high interest. Regulators of FGF23 include diet [31,32,33], PTH [34, 35], 1,25(OH)2D3 [36, 37], systemic factors such as inflammation [38,39,40,41,42], other hormones including erythropoietin (EPO) [43, 44] or insulin [45] as well as intracellular signaling pathways such as adenosine monophosphate-dependent kinase (AMPK) signaling [46].
Lactic acid is the result of anaerobic glycolysis. Its production is enhanced both under physiological conditions (e.g., physical activity above the anaerobic threshold leading to a marked surge in the plasma lactate concentration [47]) and pathological conditions (e.g., poorly controlled diabetes [48] or intoxication with metformin [49]). The resulting lactate acidosis [50] can have a wide spectrum of outcomes ranging from rapid recovery over life-threatening conditions [51] to death [52].
Since inflammation is a major driver of FGF23 production [53] and lactate induces pro-inflammatory activity [54], we sought to clarify whether and by which mechanism lactic acid regulates FGF23 production.
Methods
Cell culture
Cell culture experiments were conducted with UMR106 rat osteoblastic osteosarcoma cells (CRL-1661; ATCC, Manassas, VA, USA) cultured in Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Gibco, Life Technologies, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS) (Gibco, Life Technologies), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Life Technologies) under standard culture conditions. Cells were pretreated with 10 nM 1,25(OH)2D3 (Tocris, Bristol, UK) for 24 h (6-well format; 2 × 105 cells/well). Twenty-four hours later, they were treated with the indicated concentration of L-lactic acid or Sodium (Na+)-L-lactate (sodium chloride as vehicle control; Sigma–Aldrich, Schnelldorf, Germany; 24 h), nuclear factor kappa-light-chain enhancer of activated B-cells (NFκB) inhibitors withaferin A (Tocris; 500 nM, 24 h) or wogonin (Sigma; 100 µM, 24 h), or with vehicle only. Withaferin A and wogonin are potent inhibitors of NFκB signaling [55,56,57] that is a major enhancer of Fgf23 gene expression [58]. In further series of experiments, UMR106 cells were treated with 22.8 mM formic acid (Carl Roth, Karlsruhe, Germany), 10 mM acetic acid (Carl Roth), or water for 24 h and, pH of supernatants was measured.
Quantitative real-time PCR
Total RNA from UMR106 cells was extracted by means of RNA-Solv reagent (Omega Bio-Tek, Norcross, GA, USA). CDNA synthesis was performed with 1.2 µg RNA, random primers, and the GoScript™ Reverse Transcription System (Promega, Walldorf, Germany; 25 °C for 5 min, 42 °C for 1 h, and 70 °C for 15 min). Fgf23 expression was determined by qRT-PCR on a CFX Connect™ Real-Time System (Bio-Rad, Feldkirchen, Germany) using GoTaq qPCR Master Mix (Promega). QRT-PCR conditions were 95 °C for 2 min, 40 cycles of 95 °C for 10 s, 57 °C for 30 s, and 72 °C for 25 s (2 μl cDNA, 0.25 μM (Fgf23) or 0.5 µM (TATA box-binding protein, Tbp) of each primer, 10 μl GoTaq® Green Master Mix (Promega) and RNAse-free water up to a total volume of 20 μl). Fgf23 mRNA expression levels were referred to the expression levels of Tbp.
The following primers were used:
Rat Fgf23
-
Forward (5ʹ → 3ʹ):TAGAGCCTATTCAGACACTTC.
-
Reverse (3ʹ → 5ʹ): CATCAGGGCACTGTAGATAG.
Rat Tbp
-
Forward (5ʹ → 3ʹ): ACTCCTGCCACACCAGCC.
-
Reverse (3ʹ → 5ʹ): GGTCAAGTTTACAGCCAAGATTCA.
ELISA
To determine FGF23 in the supernatant of UMR106 cells vivaspin 6 centrifugal concentrators (Sartorius, Göttingen, Germany) were used. C-terminal FGF23 was determined by ELISA (Immutopics, San Clemente, CA, USA) according to the manufacturer’s protocol. This ELISA exhibits a sensitivity of 4 pg/ml, an intra-assay precision coefficient of variation of 4.5–6.2%, and an inter-assay precision coefficient of variation of 4.4–5.9% according to the manufacturer. With regard to the binding region of the antibodies used, homology with rat amounts to 95% (capture antibody) and 90% (detection antibody) according to the manufacturer.
Statistics
All data provided are arithmetic means ± standard error of mean (SEM), and n represents the number of independent experiments. Normality was examined with Shapiro–Wilk test. To determine statistical significance, data passing normality test were compared by paired t-test. For more than two groups, one-way analysis of variance (ANOVA) followed by Bonferroni correction was applied. Data that failed Bartlett’s test of homogeneity of variances were analyzed using Welch’s ANOVA test followed by Dunnett’s T3 correction. If Shapiro–Wilk showed p < 0.05 for comparison of more than two groups, nonparametric Kruskal–Wallis test with Dunn’s correction was used for statistical analysis. Test results with p < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism 9 (Version 9.2.0; GraphPad Software Inc., San Diego, CA, USA).
Results
Lactic acid induces FGF23 production in UMR106 cells
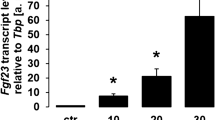
We utilized UMR106 osteoblast-like cells to study Fgf23 gene expression and FGF23 protein production. In a first series of experiments, these cells were treated with different concentrations of lactic acid for 24 h, and subsequently Fgf23 gene expression was determined by qRT-PCR. Lactic acid dose-dependently up-regulated the abundance of Fgf23 mRNA (Fig. 1) pointing to a stimulation of Fgf23 gene expression.
Lactic acid induces fibroblast growth factor 23 (Fgf23) gene expression in UMR106 cells. Arithmetic means ± SEM (n = 6) of relative Fgf23 mRNA abundance normalized to TATA box-binding protein (Tbp) expression in UMR106 cells incubated without or with lactic acid at the indicated concentration. ***p < 0.001; *p < 0.05 (Kruskal–Wallis test)
Next, we aimed to study whether the stimulatory effect of lactic acid on Fgf23 gene expression also translates into enhanced FGF23 protein secretion into the cell culture supernatant. To this end, we determined C-terminal FGF23 by ELISA. A 24 h treatment with 25 mM lactic acid significantly increased the concentration of C-terminal FGF23 in the cell culture supernatant of UMR106 cells (Fig. 2).
Sodium lactate induces Fgf23 expression in UMR106 cells
Lactic acid is a weak acid. We carried out pH measurements in the cell culture supernatant of UMR106 cells upon incubation without or with lactic acid or with other comparable weak acids, formic acid and acetic acid. As a result, a 24 h incubation of UMR106 cells without additional acid resulted in a supernatant pH of 7.44 ± 0.02 (n = 6), a value significantly different from the pH in the supernatant of cells incubated in the presence of 25 mM lactic acid (7.23 ± 0.04; n = 6; p < 0.001) or 22.8 mM formic acid (7.04 ± 0.03; n = 6; p < 0.001). In another series of experiments, a 24 h incubation without 10 mM acetic acid resulted in a supernatant pH of 7.45 ± 0.02 (n = 5), a value significantly different from the supernatant pH upon incubation with 10 mM acetic acid (7.37 ± 0.01; n = 5; p < 0.001). Since acidosis is a stimulator of FGF23 production [59, 60], we performed a new series of experiments to test whether the comparable pH-lowering effects of 25 mM lactic acid and 22.8 mM formic acid have similar effects on Fgf23 gene expression. As a result, control cells had a relative Fgf23 transcript abundance of 0.027 ± 0.001 (n = 9), a value significantly lower than in UMR106 cells incubated with 25 mM lactic acid (0.092 ± 0.005; n = 9; p < 0.05) or 22.8 mM formic acid (0.138 ± 0.012; n = 9; p < 0.001). In another series of experiments, a 24 h incubation of UMR106 cells with 10 mM acetic acid resulted in a relative Fgf23 transcript abundance of 0.021 ± 0.001 (n = 5), a value significantly higher than in control cells (0.013 ± 0.000; n = 5; p < 0.001). Thus, acidosis is likely to be a major contributor to the stimulatory effect of lactic acid on FGF23.
With Na+-lactate, no acidosis can be induced. Therefore, we performed further experiments to clarify whether Na+-lactate impacts on Fgf23. Na+-lactate also up-regulated Fgf23 gene expression in UMR106 cells within 24 h (Fig. 3), albeit to a lesser extent than lactic acid. Hence, lactate has the potential to stimulate Fgf23 gene expression even without acidosis.
Effect of lactic acid on Fgf23 expression is blunted by withaferin A and wogonin
Pro-inflammatory signaling mediated by transcription factor complex NFκB potently up-regulates FGF23 production [61], and lactic acid induces NFκB transcriptional activity [62]. Hence, we aimed to unravel whether NFκB is involved in the effect of lactic acid on FGF23. To this end, we treated UMR106 cells with and without lactic acid in the presence and absence of NFκB inhibitor withaferin A for 24 h. Withaferin A significantly blunted lactic acid-induced up-regulation of Fgf23 gene expression (Fig. 4). The same held true for wogonin, another NFκB inhibitor (Fig. 5).
The effect of lactic acid on Fgf23 gene expression is blunted by NFκB inhibitor withaferin A. Arithmetic mean ± SEM (n = 6) of relative Fgf23 mRNA abundance normalized to Tbp expression in UMR106 cells incubated with or without 25 mM lactic acid in the presence or absence of 500 nM withaferin A for 24 h. **p < 0.01 indicates significant difference from the absence of lactic acid (control). ##p < 0.01 indicates significant difference from the absence of withaferin A. (Welch’s ANOVA)
The effect of lactic acid on Fgf23 gene expression is blunted by NFκB inhibitor wogonin. Arithmetic mean ± SEM (n = 9) of relative Fgf23 mRNA abundance normalized to Tbp expression in UMR106 cells incubated with or without 25 mM lactic acid in the presence or absence of 100 µM wogonin for 24 h. *p < 0.05 indicates significant difference from the absence of lactic acid (control). ##p < 0.01 indicates significant difference from the absence of wogonin. (Kruskal–Wallis test)
Discussion
According to our study, lactic acid is a potent regulator of FGF23. This effect was, at least in part, mediated by NFκB. Lactic acid not only induced Fgf23 gene expression in UMR106 osteoblast-like cells, but also C-terminal FGF23 protein secretion into the cell culture supernatant.
A major source of lactic acid is anaerobic glycolysis [63, 64]. Physical exercise stimulates anaerobic glycolysis and, hence, lactic acid formation in working muscle [64]. If the exercise remains below the anaerobic threshold, a steady state of lactic acid production in working muscle and utilization (e.g., in the liver for gluconeogenesis [65] or in the heart for energy production) exists with the lactic acid level remaining stable [66, 67]. The anaerobic threshold is in the range of 4–5 mM lactate [68, 69]. Physical activity above the anaerobic threshold cannot be sustained for longer time [47]. According to our results, concentrations of lactic acid and lactate above the anaerobic threshold triggered enhanced FGF23 production. In line with this, exercise has been shown to stimulate FGF23 production in mice [70], and it is tempting to speculate that lactic acid contributes to FGF23 production during physical activity. In humans, one study found an increase in plasma FGF23 of participants of Giro d’Italia (road bicycle race) – no lactate values are reported [71] – while another study did not find an impact of submaximal or high-intensity exercise on FGF23 [72] although the latter study found a moderate increase in lactate during high-intensity exercise. During strenuous exercise, plasma lactate is usually in a range below 10 mM [64] although peak values of 25 mM may be reached [73]. In our study, 15 mM lactic acid and 1 mM Na+-lactate were necessary to significantly up-regulate Fgf23 gene expression. Definitely, further studies are needed to clarify whether physical exercise induces FGF23 through lactic acid in vivo.
A wide range of pathological conditions is associated with enhanced lactic acid formation causing lactic acidosis including uncontrolled diabetes mellitus [48] or, as a rare but dangerous adverse effect, metformin [49]. Lactic acidosis is a very serious condition as illustrated by a fatality rate of 25–50% in metformin-associated lactic acidosis [48, 49, 74]. In the latter case, the mean lactate concentrations may be 23 mM with some values as high as 35 mM [49, 75]. These concentrations are in the range of the highest lactic acid concentrations applied in our in vitro study. This supports the notion that lactic acid may be a relevant stimulator of FGF23 production also in vivo, at least in pathological lactic acidosis. As higher FGF23 levels are associated with poorer outcome in several disorders including kidney and cardiovascular diseases [27], higher FGF23 in severe lactic acidosis may also be indicative of a dismal prognosis. Moreover, severe acidosis worsens outcome in CKD [76] and higher FGF23 levels are associated with poorer outcome in this disorder [77]. Hence, normalizing plasma pH may also prove efficient in CKD due to the lowering of FGF23. Clearly, clinical studies are needed to address this question.
Acidosis is also a very common consequence of CKD [52]. Moreover, metformin-induced lactic acidosis typically affects patients with severe CKD [78]. Since FGF23 plasma levels go up early in CKD and predict prognosis [26, 79], lactic acid-induced FGF23 production may also be a mechanism relevant in CKD.
Addition of lactic acid caused a small but significant decrease in pH. Since acidosis has already been demonstrated to induce FGF23 production [59], we considered that the effect of lactic acid on FGF23 was, at least in part, due to acidosis. In line with this, formic acid or acetic acid induced a pH drop while stimulating Fgf23 gene expression. However, also Na+-lactate, which is a weak base, was capable of enhancing Fgf23. Hence, cellular acidosis clearly contributes to lactic acid-induced FGF23 production, but may not fully explain it.
We could significantly blunt the stimulatory effect of lactic acid on FGF23 with two different inhibitors of NFκB, wogonin and withaferin A, pointing to an involvement of NFκB. In line with this, lactate is a stimulator of NFκB activity [62], and on the other hand, NFκB and inflammation have been demonstrated to be important inducers of FGF23 formation [39, 58].
Conclusion
Taken together, our study demonstrates that lactic acid induces Fgf23 gene expression and protein synthesis in vitro at concentrations encountered in vivo in lactic acidosis. This effect is, at least in part, mediated by NFκB and acidosis. High FGF23 concentrations in lactic acidosis may be suggestive for poor prognosis, although clinical studies are needed for clarification.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- 1,25(OH)2D3 :
-
Calcitriol
- AMPK:
-
Adenosine monophosphate-dependent kinase
- ANOVA:
-
Analysis of variance
- DMEM:
-
Dulbecco’s modified eagle medium
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPO:
-
Erythropoietin
- FBS:
-
Fetal bovine serum
- FGF23:
-
Fibroblast growth factor 23
- NFκB:
-
Nuclear factor kappa-light-chain enhancer of activated B-cells
- PTH:
-
Parathyroid hormone
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- sKL:
-
Soluble klotho
- SEM:
-
Standard error of mean
- Tbp:
-
TATA box-binding protein
References
Edmonston D, Wolf M (2020) FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol 16:7–19. https://doi.org/10.1038/s41581-019-0189-5
Erben RG (2018) Physiological actions of fibroblast growth factor-23. Front Endocrinol (Lausanne) 9:267. https://doi.org/10.3389/fendo.2018.00267
Leifheit-Nestler M, Haffner D (2018) Paracrine effects of FGF23 on the heart. Front Endocrinol (Lausanne) 9:278. https://doi.org/10.3389/fendo.2018.00278
White KE, Evans EW, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux et al (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348. https://doi.org/10.1038/81664
Bär L, Stournaras C, Lang F, Föller M (2019) Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett 593:1879–1900. https://doi.org/10.1002/1873-3468.13494
Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD (2006) Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17:1305–1315. https://doi.org/10.1681/ASN.2005111185
Mace ML, Olgaard K, Lewin E (2020) New aspects of the kidney in the regulation of fibroblast growth factor 23 (FGF23) and mineral homeostasis. Int J Mol Sci. https://doi.org/10.3390/ijms21228810
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T et al (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568. https://doi.org/10.1172/JCI19081
Avcioglu G, Özbek Ipteç B, Akcan G, Görgün B, Fidan K, Carhan A et al (2020) Effects of 1,25-Dihydroxy vitamin D3 on TNF-α induced inflammation in human chondrocytes and SW1353 cells: a possible role for toll-like receptors. Mol Cell Biochem 464:131–142. https://doi.org/10.1007/s11010-019-03655-z
Vervloet M (2019) Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 15:109–120. https://doi.org/10.1038/s41581-018-0087-2
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M et al (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008. https://doi.org/10.1172/JCI32409
Goltzman D, Mannstadt M, Marcocci C (2018) Physiology of the calcium-parathyroid hormone-vitamin D axis. Front Horm Res 50:1–13. https://doi.org/10.1159/000486060
Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV et al (2007) Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27:3417–3428. https://doi.org/10.1128/MCB.02249-06
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP et al (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123. https://doi.org/10.1074/jbc.C500457200
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317:803–806. https://doi.org/10.1126/science.1143578
Chen T-H, Kuro-o M, Chen C-H, Sue Y-M, Chen Y-C, Wu H-H, Cheng C-Y (2013) The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698:67–73. https://doi.org/10.1016/j.ejphar.2012.09.032
Mencke R, Olauson H, Hillebrands J-L (2017) Effects of Klotho on fibrosis and cancer: a renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev 121:85–100. https://doi.org/10.1016/j.addr.2017.07.009
Chen Y, Huang C, Zhu S-Y, Zou H-C, Xu C-Y, Chen Y-X (2021) Overexpression of HOTAIR attenuates Pi-induced vascular calcification by inhibiting Wnt/β-catenin through regulating miR-126/Klotho/SIRT1 axis. Mol Cell Biochem 476:3551–3561. https://doi.org/10.1007/s11010-021-04164-8
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833. https://doi.org/10.1126/science.1112766
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S et al (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90:985–996. https://doi.org/10.1016/j.kint.2016.05.019
Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C et al (2015) Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22:1020–1032. https://doi.org/10.1016/j.cmet.2015.09.002
Böckmann I, Lischka J, Richter B, Deppe J, Rahn A, Fischer D-C et al (2019) FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. https://doi.org/10.3390/ijms20184634
Rossaint J, Oehmichen J, van Aken H, Reuter S, Pavenstädt HJ, Meersch M et al (2016) FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126:962–974. https://doi.org/10.1172/JCI83470
Chu C, Elitok S, Zeng S, Xiong Y, Hocher C-F, Hasan AA et al (2021) C-terminal and intact FGF23 in kidney transplant recipients and their associations with overall graft survival. BMC Nephrol 22:125. https://doi.org/10.1186/s12882-021-02329-7
Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A et al (2007) Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) Study. J Am Soc Nephrol 18:2600–2608. https://doi.org/10.1681/ASN.2006080936
Lu X, Hu MC (2017) Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis (Basel) 3:15–23. https://doi.org/10.1159/000452880
Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE (2013) Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol 8:781–786. https://doi.org/10.2215/CJN.09570912
Shah NH, Dong C, Elkind MSV, Sacco RL, Mendez AJ, Hudson BI et al (2015) Fibroblast growth factor 23 is associated with carotid plaque presence and area: the northern manhattan study. Arterioscler Thromb Vasc Biol 35:2048–2053. https://doi.org/10.1161/ATVBAHA.115.305945
Di Giuseppe R, Kühn T, Hirche F, Buijsse B, Dierkes J, Fritsche A et al (2015) Plasma fibroblast growth factor 23 and risk of cardiovascular disease: results from the EPIC-Germany case-cohort study. Eur J Epidemiol 30:131–141. https://doi.org/10.1007/s10654-014-9982-4
Ferrari SL, Bonjour J-P, Rizzoli R (2005) Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524. https://doi.org/10.1210/jc.2004-1039
Perwad F, Azam N, Zhang MYH, Yamashita T, Tenenhouse HS, Portale AA (2005) Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146:5358–5364. https://doi.org/10.1210/en.2005-0777
Shafie A, Rahimi AM, Ahmadi I, Nabavizadeh F, Ranjbaran M, Ashabi G (2020) High-protein and low-calorie diets improved the anti-aging Klotho protein in the rats’ brain: the toxic role of high-fat diet. Nutr Metab (London) 17:86. https://doi.org/10.1186/s12986-020-00508-1
Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y (2007) Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18:2683–2688. https://doi.org/10.1681/ASN.2006070783
Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T (2011) Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49:636–643. https://doi.org/10.1016/j.bone.2011.06.025
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR et al (2005) 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042. https://doi.org/10.1152/ajpgi.00243.2005
Saini RK, Kaneko I, Jurutka PW, Forster R, Hsieh A, Hsieh J-C et al (2013) 1,25-dihydroxyvitamin D(3) regulation of fibroblast growth factor-23 expression in bone cells: evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif Tissue Int 92:339–353. https://doi.org/10.1007/s00223-012-9683-5
Bansal S, Friedrichs WE, Velagapudi C, Feliers D, Khazim K, Horn D et al (2017) Spleen contributes significantly to increased circulating levels of fibroblast growth factor 23 in response to lipopolysaccharide-induced inflammation. Nephrol Dial Transplant 32:960–968. https://doi.org/10.1093/ndt/gfw376
David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V et al (2016) Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89:135–146. https://doi.org/10.1038/ki.2015.290
Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH et al (2012) Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7:1155–1162. https://doi.org/10.2215/CJN.13281211
Sato H, Kazama JJ, Murasawa A, Otani H, Abe A, Ito S et al (2016) Serum fibroblast growth factor 23 (FGF23) in patients with rheumatoid arthritis. Intern Med 55:121–126. https://doi.org/10.2169/internalmedicine.55.5507
Czaya B, Faul C (2019) The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. https://doi.org/10.3390/ijms20174195
Daryadel A, Bettoni C, Haider T, Imenez Silva PH, Schnitzbauer U, Pastor-Arroyo EM et al (2018) Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch 470:1569–1582. https://doi.org/10.1007/s00424-018-2171-7
Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA et al (2017) Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 102:e427–e430. https://doi.org/10.3324/haematol.2017.167882
Bär L, Feger M, Fajol A, Klotz L-O, Zeng S, Lang F et al (2018) Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci U S A 115:5804–5809. https://doi.org/10.1073/pnas.1800160115
Glosse P, Feger M, Mutig K, Chen H, Hirche F, Hasan AA et al (2018) AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int 94:491–501. https://doi.org/10.1016/j.kint.2018.03.006
Ghosh AK (2004) Anaerobic threshold: its concept and role in endurance sport. Malays J Med Sci 11:24–36
Cox K, Cocchi MN, Salciccioli JD, Carney E, Howell M, Donnino MW (2012) Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care 27:132–137. https://doi.org/10.1016/j.jcrc.2011.07.071
Boucaud-Maitre D, Ropers J, Porokhov B, Altman J-J, Bouhanick B, Doucet J et al (2016) Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med 33:1536–1543. https://doi.org/10.1111/dme.13098
Lee Y-J, Nam H-S, Cho M-K, Lee S-H (2020) Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis. Mol Cell Biochem 467:45–56. https://doi.org/10.1007/s11010-020-03699-6
Hudak SK, Overkamp D, Wagner R, Häring H-U, Heni M (2015) Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J 14:117. https://doi.org/10.1186/s12937-015-0076-2
Kraut JA, Madias NE (2014) Lactic acidosis. N Engl J Med 371:2309–2319. https://doi.org/10.1056/nejmra1309483
Egli-Spichtig D, Imenez Silva PH, Glaudemans B, Gehring N, Bettoni C, Zhang MYH et al (2019) Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non-renal inflammation. Kidney Int 96:890–905. https://doi.org/10.1016/j.kint.2019.04.009
Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E et al (2019) Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T cell metabolic rewiring. Cell Metab 30:1055-1074.e8. https://doi.org/10.1016/j.cmet.2019.10.004
Heyninck K, Lahtela-Kakkonen M, van der Veken P, Haegeman G, Vanden BW (2014) Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol 91:501–509. https://doi.org/10.1016/j.bcp.2014.08.004
Tang N-Y, Yang J-S, Chang Y-H, Lu H-F, Hsia T-C, Lin W-C, Chung J-G (2006) Effects of wogonin on the levels of cytokines and functions of leukocytes associated with NF-kappa B expression in Sprague-Dawley rats. In Vivo 20:527–532
Xu X, Zhang X, Zhang Y, Yang L, Liu Y, Huang S et al (2017) Wogonin reversed resistant human myelogenous leukemia cells via inhibiting Nrf2 signaling by Stat3/NF-κB inactivation. Sci Rep 7:39950. https://doi.org/10.1038/srep39950
Zhang B, Yan J, Umbach AT, Fakhri H, Fajol A, Schmidt S et al (2016) NFκB-sensitive Orai1 expression in the regulation of FGF23 release. J Mol Med (Berl) 94:557–566. https://doi.org/10.1007/s00109-015-1370-3
Krieger NS, Culbertson CD, Kyker-Snowman K, Bushinsky DA (2012) Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am J Physiol Renal Physiol 303:F431–F436. https://doi.org/10.1152/ajprenal.00199.2012
Liu D, Alvarez-Elías AC, Wile B, Belostotsky V, Filler G (2017) Deviations from the expected relationship between serum FGF23 and other markers in children with CKD: a cross-sectional study. BMC Nephrol 18:204. https://doi.org/10.1186/s12882-017-0623-5
Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A et al (2015) Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol 399:208–218. https://doi.org/10.1016/j.mce.2014.10.007
Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y (2009) Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol 182:2476–2484. https://doi.org/10.4049/jimmunol.0802059
Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287:R502–R516. https://doi.org/10.1152/ajpregu.00114.2004
Spurway NC (1992) Aerobic exercise, anaerobic exercise and the lactate threshold. Br Med Bull 48:569–591. https://doi.org/10.1093/oxfordjournals.bmb.a072564
Scott CB (2005) Contribution of anaerobic energy expenditure to whole body thermogenesis. Nutr Metab (London) 2:14. https://doi.org/10.1186/1743-7075-2-14
Sun S, Li H, Chen J, Qian Q (2017) Lactic acid: no longer an inert and end-product of glycolysis. Physiology (Bethesda) 32:453–463. https://doi.org/10.1152/physiol.00016.2017
Gastin PB (2001) Energy system interaction and relative contribution during maximal exercise. Sports Med 31:725–741. https://doi.org/10.2165/00007256-200131100-00003
Kindermann W, Simon G, Keul J (1979) The significance of the aerobic-anaerobic transition for the determination of work load intensities during endurance training. Eur J Appl Physiol Occup Physiol 42:25–34. https://doi.org/10.1007/BF00421101
Płoszczyca K, Jazic D, Piotrowicz Z, Chalimoniuk M, Langfort J, Czuba M (2020) Comparison of maximal lactate steady state with anaerobic threshold determined by various methods based on graded exercise test with 3-minute stages in elite cyclists. BMC Sports Sci Med Rehabil 12:70. https://doi.org/10.1186/s13102-020-00219-3
Li D-J, Fu H, Zhao T, Ni M, Shen F-M (2016) Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism 65:747–756. https://doi.org/10.1016/j.metabol.2016.02.009
Lombardi G, Corsetti R, Lanteri P, Grasso D, Vianello E, Marazzi MG et al (2014) Reciprocal regulation of calcium-/phosphate-regulating hormones in cyclists during the Giro d’Italia 3-week stage race. Scand J Med Sci Sports 24:779–787. https://doi.org/10.1111/sms.12080
Emrich IE, Baier M, Zawada AM, Meyer T, Fliser D, Scharhag J, Heine GH (2019) Plasma FGF23 does not rise during physical exercise as a physiological model of sympathetic activation. Clin Res Cardiol 108:341–343. https://doi.org/10.1007/s00392-018-1347-7
Withers RT, Sherman WM, Clark DG, Esselbach PC, Nolan SR, Mackay MH, Brinkman M (1991) Muscle metabolism during 30, 60 and 90 s of maximal cycling on an air-braked ergometer. Eur J Appl Physiol Occup Physiol 63:354–362. https://doi.org/10.1007/BF00364462
Kajbaf F, Lalau J-D (2014) Mortality rate in so-called “metformin-associated lactic acidosis”: a review of the data since the 1960s. Pharmacoepidemiol Drug Saf 23:1123–1127. https://doi.org/10.1002/pds.3689
Kajbaf F, Lalau J-D (2013) The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol 14:22. https://doi.org/10.1186/2050-6511-14-22
Chen W, Abramowitz MK (2014) Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol 15:55. https://doi.org/10.1186/1471-2369-15-55
Isakova T (2012) Fibroblast growth factor 23 and adverse clinical outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 21:334–340. https://doi.org/10.1097/MNH.0b013e328351a391
Lazarus B, Wu A, Shin J-I, Sang Y, Alexander GC, Secora A et al (2018) Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 178:903–910. https://doi.org/10.1001/jamainternmed.2018.0292
Zeng S, Querfeld U, Feger M, Haffner D, Hasan AA, Chu C et al (2020) Relationship between GFR, intact PTH, oxidized PTH, non-oxidized PTH as well as FGF23 in patients with CKD. FASEB J 34:15269–15281. https://doi.org/10.1096/fj.202000596R
Acknowledgements
The authors thank M. Feger for experimental support and H. Froß, C. Heidel, and A. Ullrich for technical help. This work was supported by the Deutsche Forschungsgemeinschaft [Fo 695/2-2].
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Deutsche Forschungsgemeinschaft [Fo 695/2-2].
Author information
Authors and Affiliations
Contributions
MF designed the research. MF and JA interpreted data and wrote the manuscript; JA performed the research and analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors state they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alber, J., Föller, M. Lactic acid induces fibroblast growth factor 23 (FGF23) production in UMR106 osteoblast-like cells. Mol Cell Biochem 477, 363–370 (2022). https://doi.org/10.1007/s11010-021-04287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04287-y