Abstract
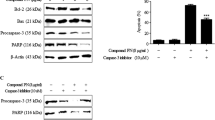
Arctigenin, a mitochondrial complex I inhibitor, has been identified as a potential anti-tumor agent, but the involved mechanism still remains elusive. Herein, we studied the underlying mechanism(s) of action of arctigenin on acidity-tolerant prostate cancer PC-3AcT cells in the lactic acid-containing medium. At concentration showing no toxicity on normal prostate epithelial RWPE-1 and HPrEC cells, arctigenin alone or in combination with docetaxel induced significant cytotoxicity in PC-3AcT cells compared to parental PC-3 cells. With arctigenin treatment, reactive oxygen species (ROS) levels, annexin V-PE positive fractions, sub-G0/G1 peak in cell cycle analysis, mitochondrial membrane depolarization, and cell communication network factor 1 (CCN1) levels were increased, while cellular ATP content and phospho (p)-Akt level were decreased. Pretreatment with ROS scavenger N-acetylcysteine effectively reversed the series of phenomena caused by arctigenin, suggesting that ROS served as upstream molecules of arctigenin-driven cytotoxicity. Meanwhile, arctigenin increased the levels of p-receptor-interacting serine/threonine-protein kinase 3 (p-RIP3) and p-mixed lineage kinase domain-like pseudokinase (p-MLKL) as necroptosis mediators, and pretreatment with necroptosis inhibitor necrostatin-1 restored their levels and cell viability. Treatment of spheroids with arctigenin resulted in necroptotic cell death, which was prevented by N-acetylcysteine. The siRNA-based knockdown of CCN1 suppressed the levels of MLKL, B-cell lymphoma 2 (Bcl-2), and induced myeloid leukemia cell differentiation (Mcl-1) with increased cleavage of Bcl-2-associated X (Bax) and caspase-3. Collectively, these results provide new insights into the molecular mechanisms underlying arctigenin-induced cytotoxicity, and support arctigenin as a potential therapeutic agent for targeting non-Warburg phenotype through induction of necroptosis via ROS-mediated mitochondrial damage and CCN1 upregulation.






Similar content being viewed by others
References
Vanlangenakker N, Vanden Berghe T, Vandenabeele P (2012) Many stimuli pull the necrotic trigger, an overview. Cell Death Differ 19(1):75–86
Ke B, Tian M, Li J et al (2016) Targeting programmed cell death using small-molecule compounds to improve potential cancer therapy. Med Res Rev 36(6):983–1035
Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370(5):455–465
Shivapurkar N, Reddy J, Chaudhary PM et al (2003) Apoptosis and lung cancer: a review. J Cell Biochem 88(5):885–898
Chen D, Yu J (1865) Zhang L (2016) Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta 2:228–236
Cohen MB, Rokhlin OW (2009) Mechanisms of prostate cancer cell survival after inhibition of AR expression. J Cell Biochem 106(3):363–371
Li QC, Liang Y, Tian Y et al (2016) Arctigenin induces apoptosis in colon cancer cells through ROS/p38MAPK pathway. J BUON 21(1):87–94
Hsieh CJ, Kuo PL, Hsu YC et al (2014) Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol Med 67(2):159–170
He Y, Fan Q, Cai T et al (2018) Molecular mechanisms of the action of arctigenin in cancer. Biomed Pharmacother 108:403–407
Wang P, Solorzano W, Diaz T et al (2017) Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin Nutr Exp 13:1–11
Wang P, Phan T, Gordon D et al (2015) Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol Nutr Food Res 59(2):250–261
Lu Z, Cao S, Zhou H et al (2015) Mechanism of arctigenin-induced specific cytotoxicity against human hepatocellular carcinoma cell lines: Hep G2 and SMMC7721. PLoS ONE 10(5):e0125727
Wang P, Wang B, Chung S et al (2014) Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv 4(66):35242–35250
Gu Y, Qi C, Sun X et al (2012) Arctigenin preferentially induces tumor cell death under glucose deprivation by inhibiting cellular energy metabolism. Biochem Pharmacol 84(4):468–476
Walenta S, Mueller-Klieser WF (2004) Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 14(3):267–274
Denko NC (2008) Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8(9):705–713
Raghunand N, Gillies RJ (2000) pH and drug resistance in tumors. Drug Resist Update 3(1):39–47
Lee YJ, Oh JE, Lee SH (2018) Arctigenin shows preferential cytotoxicity to acidity-tolerant prostate carcinoma PC-3 cells through ROS-mediated mitochondrial damage and the inhibition of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun 505(4):1244–1250
Chambers KF, Mosaad EM, Russell PJ et al (2014) 3D Cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer. PLoS ONE 9(11):e111029
Wu H, Ying M, Hu X (2016) Lactic acidosis switches cancer cells from aerobic glycolysis back to dominant oxidative phosphorylation. Oncotarget 7(26):40621–40629
Huang SL, Yu RT, Gong J et al (2012) Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia 55(5):1469–1481
Bleau AM, Planque N, Perbal B (2005) CCN proteins and cancer: two to tango. Front Biosci 10:998–1009
Chen CC, Lau LF (2009) Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol 41(4):771–783
Bai T, Chen CC, Lau LF (2010) Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. Immunol 184(6):3223–3232
Juric V, Chen CC, Lau LF (2009) Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Mol Cell Biol 29(12):3266–3279
Krysko O, Aaes TL, Kagan VE et al (2017) Necroptotic cell death in anti-cancer therapy. Immunol Rev 280(1):207–219
Chen CC, Young JL, Monzon RI et al (2007) Cytotoxicity of TNFα is regulated by integrin-mediated matrix signaling. EMBO J 26(5):1257–1267
Schenk B, Fulda S (2015) Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene 34(47):5796–5806
Wood DE, Newcomb EW (2000) Cleavage of Bax enhances its cell death function. Exp Cell Res 256(2):375–382
van Waveren C, Sun Y, Cheung HS et al (2006) Oxidative phosphorylation dysfunction modulates expression of extracellular matrix–remodeling genes and invasion. Carcinogenesis 27(3):409–418
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education (No. NRF-2017R1D1A3B04031891).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, YJ., Nam, HS., Cho, MK. et al. Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis. Mol Cell Biochem 467, 45–56 (2020). https://doi.org/10.1007/s11010-020-03699-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03699-6