Abstract
Purpose
Habitat connectivity is integral to current biodiversity science and conservation strategies. Originally, the connectivity concept stressed the role of individual movements for landscape-scale processes. Connectivity determines whether populations can survive in sub-optimal patches (i.e., source-sink effects), complete life cycles relying on different habitat types (i.e., landscape complementation), and benefit from supplementary resources distributed over the landscape (i.e., landscape supplementation). Although the past decades have witnessed major improvements in habitat connectivity modeling, most approaches have yet to consider the multiplicity of habitat types that a species can benefit from. Without doing so, connectivity analyses potentially fail to meet one of their fundamental purposes: revealing how complex individual movements lead to landscape-scale ecological processes.
Methods
To bridge this conceptual and methodological gap, we propose to include multiple habitat types in spatial graph models of habitat connectivity, where nodes traditionally represent a single habitat type. Multiple habitat graphs will improve how we model connectivity and related landscape ecological processes, and how they are impacted by land cover changes.
Results
In three case studies, we use these graphs to model (i) source-sink effects, (ii) landscape supplementation, and (iii) complementation processes, in urban ecosystems, agricultural landscapes, and amphibian habitat networks, respectively. A new version of the Graphab open-source software implements the proposed approach.
Conclusion
Multiple habitat graphs help address crucial conservation challenges (e.g., urban sprawl, biological control, climate change) by representing more accurately the dynamics of populations, communities, and their interactions. Our approach thereby extends the ecologist’s toolbox and aims at fostering the alignment between landscape ecology theory and practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Landscape ecology has been key for unraveling the influence of landscape structure on ecological processes and resulting biodiversity patterns (Turner 1989). It revealed how the heterogeneity of land cover and habitat types provides species with a variety of resources, and even allows them to colonize and survive in sub-optimal patches located nearby source patches. After Dunning et al. (1992) stressed the roles of landscape complementation, landscape supplementation, and source-sink effects, Taylor et al. (1993) relevantly pointed out that these processes tightly depend on species ability to move across the landscape, thereby giving birth to the landscape connectivity concept. This concept has first been widely acknowledged by ecologists as an overall landscape property (Forman 1995). However, it was then recognized that it should rather be associated with a given habitat type and/or a given species or taxon, and analyzed accordingly (Taylor et al. 2006). Habitat connectivity analyses are nowadays integrated into most conservation strategies (Crooks and Sanjayan 2006), and prove helpful for understanding the influence of landscape and habitat structures on species responses (Baguette et al. (2013), Gonzalez et al. (2017), Resasco (2019), among others).
Meanwhile, however, three landscape ecological processes stressed by Dunning et al. (1992) and brought forth by connectivity (i.e., source-sink effects, landscape supplementation, and landscape complementation) have often been overlooked in connectivity analyses. Indeed, modeling approaches commonly disregard the fact that some species rely on different habitat types to complete their life-cycle, moving between them according to seasonal, transient, or permanent habitat changes, or over multi-generational time scales (but see Haase et al. (2017) and Mims et al. (2023)). For example, dispersal movements between several types of habitat can make it possible for some species to persist in apparently suboptimal habitats, such as urban or other human-made habitats (Snep et al. 2006). Besides, while many natural enemies feed on pest species that seasonally peak in crops, they reproduce and overwinter in semi-natural habitats (Schellhorn et al. 2014; Gurr et al. 2017). Similarly, many amphibian species reproduce in wet areas while overwintering in forest areas (Cayuela et al. 2020). Consequently, failing to account for the heterogeneity of habitats and related connectivity patterns could limit our ability to properly understand biodiversity dynamics and design adequate conservation strategies.
The lack of consideration of such heterogeneity is partly explained by methodological shortcomings. Commonly used approaches for modeling habitat connectivity include pixel-based methods computing least-cost paths or resistance distances to locate potential movement areas (McRae 2006). Modelers often combine the identification of movement paths with the delineations of discrete habitat patches, and eventually represent habitat patch networks as landscape graphs (Galpern et al. 2011). For the latter approach, some authors have developed methods to analyze connectivity while considering land cover changes between several time periods (Martensen et al. 2017; Uroy et al. 2021). Yet, connectivity modeling tools available for these temporal analyses do not make it possible to distinguish several habitat types and the variation of movement types and timing between each of them. A modeling tool tailored to fit these cases will allow modelers to represent more realistically the processes at play. This could additionally reveal the importance of a given type of habitat patch for species’ life stages occurring in other types of habitat patches. Finally, this will help in the design of conservation measures that increase the amount and reachability of one type of habitat while indirectly benefiting biodiversity in another type of habitat. Similarly, such a modeling process could assess whether conservation strategies for a given habitat will negatively impact the connectivity of other habitats and their networks. Such an approach thus appears timely from both theoretical and applied perspectives.
To bridge these gaps, we propose an extension of the landscape graph modeling approach introduced by Urban and Keitt (2001), that aims to represent the connectivity of habitat networks made of different types of habitats. In an effort to foster the use of this approach, we released a new version of Graphab, an open-source software program (Foltête et al. 2012, 2021). Graphab 3.0 will allow ecologists to use multiple habitat graphs for analyzing connectivity and revealing the landscape-scale processes driving their biological responses of interest. In the following sections, we first present the theoretical underpinnings of this modeling approach. Next, we present how we adapted commonly used graph-based connectivity analyses and metrics (Rayfield et al. 2011) to multiple habitat graphs. We then illustrate the value of multiple habitat graphs through three case studies associated with an increasing diversity of modeled habitats and movement types. These examples showcase how to (i) consider potential source-sink dynamics for managing urban biodiversity, (ii) improve semi-natural area management as part of biological control strategies, and (iii) consider seasonal migrations for conserving composite habitat networks. We finally pinpoint future promising applications and developments of this new approach.
Background
Species need to move to accomplish different stages of their life cycles (e.g., foraging, breeding, overwintering), colonize new sites, extend their ranges or disperse between different populations (Schlägel et al. 2020). Some species occupy discrete habitat patches across the landscape, often after these habitats have been reduced and subdivided by human activities. The dynamics of these discrete populations and their dependence on species movements have been better understood following pioneering works on island biogeography theory (MacArthur and Wilson 1967), spreading of risk (Den Boer 1968), and metapopulation theory (Levins 1969, 1970; Hanski 1989). This body of research served as a basis for the connectivity concept, coined by Taylor et al. (1993). By allowing individuals to move across the landscape, habitat connectivity brings forth landscape-scale ecological processes, and is responsible for the fact that, in the words of Dunning et al. (1992), "the sum of the parts of a landscape will likely not add up to the observed whole". Indeed, individuals’ movements give rise to the emergence of several types of spatial dynamics, depending on the types of habitat, resources, and movements involved (Fig. 1). Furthermore, because the quality and spatial distribution of habitats and resources change through time, these processes also possess their own temporal dynamics, largely driven by human activities and their consequences (e.g., land cover and climate changes). These spatial and temporal dynamics emerge from the structure of spatial networks made of habitat patches connected to different degrees depending on landscape composition and configuration (Nicoletti et al. 2023; Savary et al. 2024).
Connectivity modeling aims to map potential movements between habitat patches accurately, either for providing patch-scale or landscape-scale predictors of biological responses or for the design of spatially-explicit conservation strategies. Over the past decades, graph theory has been particularly helpful to study habitat patch networks at multiple scales and understand their ecological consequences (Keitt et al. 1997; Borthagaray et al. 2014). Landscape graph modeling has been the cornerstone of these graph-based frameworks, which represents habitat networks as sets of nodes featuring habitat patches connected by links supposed to match potential movement paths (Keitt et al. 1997; Urban and Keitt 2001; Galpern et al. 2011).
We propose to further extend landscape graph modeling by accounting for different types of nodes and links corresponding to different types of habitats/resources and movement paths, respectively (Fig. 1). Including an additional level of spatiotemporal heterogeneity in the modeling will represent more realistically the dynamics of the following landscape-scale ecological processes: (i) source-sink effects, (ii) landscape supplementation, and (iii) landscape complementation. When modeling habitat patch networks as multiple habitat graphs, we assume that the spatial dynamics of the latter processes takes place on the components of the multiple habitat graph, i.e., its nodes and links (Fortin et al. 2021). In contrast, temporal dynamics are modeled by seasonal or permanent modifications of these graph components, which reflect the dynamics of the graph itself. Although Figure 1 and the examples below only consider two habitat types, multiple habitat graphs can also model cases in which these ecological processes depend on more than two habitats.
Multiple habitat graphs (center-right column) provide an advantage over single habitat graphs (center-left column) to model how the connectivity between different types of habitats and different movement paths brings forth the following landscape-scale ecological processes: (a) source-sink effects, (b) landscape supplementation, and (c) landscape complementation (vertically separated frames). Habitat patches (nodes) and movement paths (links) are represented by plain circles or thick lines, respectively; with colors specifying the habitat/resource types and movement types they correspond to, for multiple habitat graphs (single colored otherwise). The right-most column provides application examples (cf. Case studies section) where the multiple habitat graph approach will prove helpful. Although the chosen examples only include two habitat types, our approach and the new version of Graphab allow the consideration of more than two habitats in multiple habitat graphs
Source-sink effects
Source-sink effects allow populations to survive in patches that cannot sustain long term positive growth rates without benefiting from migrant inflow (i.e., net importers of individuals) which originate from source patches (Pulliam 1988; Loreau et al. 2013). Beyond its dependency on patch quality, this landscape-scale process depends on the spatial configuration of source and sink patches as well as on the permeability of the matrix. We can expect this process to affect population demographics and community diversity in a wide range of contexts where patches of habitats of the same nature exhibit highly heterogeneous qualities (Mouquet and Loreau 2003). For instance, in agricultural landscapes, regularly ploughed and/or fertilized temporary grasslands can be sink patches for insect or plant species that do not survive these practices but that can recolonize them from permanent grasslands, which thereby act as source patches.
To model how source-sink effects impact population dynamics, multiple habitat graphs make it possible to distinguish habitat patches of different quality, as well as different types of movement paths connecting them (i.e., source-source, sink-sink, and source-sink links; Fig. 1a). They model the connectivity between source and sink patches in a more realistic way than by considering that, despite their different areas and relative isolation, all the patches and all the movement paths among them are of equivalent nature. For instance, the relative proportion of links between source and sink patches, and their suitability for movements, can have substantial ecological consequences, and is therefore worth quantifying. They can also model how changes in land use or patch quality affect ecological processes to varying degrees depending on the type of patches and paths they modify. The destruction of either source patches or movement paths between source and sink patches could, for example, affect the demography of sink patches in a substantial way. On the contrary, one can expect the destruction of sink patches, or movement paths among sink patches, to affect the overall population demographics to a much lesser extent.
Importantly, distinguishing source and sink patches, i.e., patches that are net exporter/importer of individuals or propagules (Pulliam (1988); see Loreau et al. (2013) for a discussion on the conditionality of this definition), is a key step in the modeling of multiple habitat graphs representing source-sink dynamics. We first recommend using this approach when discrete habitat types, occupied by similar species and having the same structure yet differing in quality, can be distinguished and modeled as distinct habitat types. These quality differences can potentially turn them into sources and sinks for a given species, regardless of their area or other parameters accounted for in single habitat graphs; e.g., wooded urban habitat patches and peri-urban forests (Fig. 1a). When accurate demographic data are not available to estimate growth rates but some habitat types are suspected to act as sources and sinks (Lepczyk et al. 2017; Stillfried et al. 2017), confronting other empirical data to the metrics either derived from a single or multiple habitat graph can help test whether source-sink dynamics are happening. Finally, note that a discrete, and rather arbitrary, classification of habitat patches can be assumed in order to quantify the connectivity between habitat types that are either highly affected by human activities, or subject to more natural dynamics. For example, such a distinction could help deriving separate estimates for the connectivity (i) among habitat patches located within protected areas, (ii) among other structurally similar habitat patches, and (iii) stemming from their inter-connections.
Landscape supplementation
Landscape supplementation processes take place when the population occupying a focal patch increases in response to the availability of additional, and often substitutable, resources in nearby patches of the same or of a different habitat type (Dunning et al. 1992). The focal patch is often heterogeneous and provides all the resources needed for the species’ life cycle. Additional nearby patches can include one or several similar or substitutable resources to supplement the focal patch resources. In some cases, these nearby patches might not be suitable by themselves and would thus only be used during a short period (e.g., for foraging on highly seasonal and/or patchy resources). In that respect, this process differs from source-sink dynamics in that it emerges from periodic movements of individuals to supplementary resources, and not from dispersal exchanges between equivalent patches affecting their net demographic balance. For instance, some bird species can form large populations in small woodlots because their individuals also forage in surrounding forests (Whitcomb et al. 1977). Similarly, some insect populations breed in field margins and maintain large populations by foraging in crops (Fig. 1b, and second case study).
Multiple habitat graphs can differentiate patches sustaining populations over their entire life cycle from patches only providing supplementary resources in a facultative and occasional way. They also distinguish the movement paths between these different types of habitat patches and can account for differences in the movement behaviors adopted for foraging, breeding, or dispersing. Note that single habitat graphs already made it possible to model this process when it happens between patches of the same type, as soon as periodic and short foraging movements were assumed in the modeling. However, multiple habitat graphs extend this to cases in which individuals supplement their resources in patches of different and distinguishable types. Based on the above, this can improve our ability to explain population sizes or predict predation pressure in nearby patches, for instance. Additionally, one can build these graphs for several dates by updating resource distributions as a function of land use changes. This provides better insights into the temporal dynamics of populations in complex and evolving landscapes. Figure 1b and a subsequent case study illustrate the use of such an approach for modeling biocontrol in agricultural landscapes.
Landscape complementation
Some species need to move to different habitat types throughout their whole life cycle to forage, breed, or overwinter. Consequently, they only survive in landscapes that provide these different types of habitats and are permeable enough to the movements between them. This defines the landscape-scale ecological process called "landscape complementation" (Dunning et al. 1992). Most amphibians are highly dependent on such processes (Fig. 1c, and third case study), alike many aquatic species that separate their breeding habitats from other habitats (e.g., fish species spawning in flooded grasslands). However, several exclusively terrestrial taxa can also exhibit such a movement pattern, making them dependent on the connectivity of complementary habitats. For example, the lesser horseshoe bat (Rhinolophus hipposideros) differentiates foraging, roosting, and swarming sites. Given the sensitivity of this species to habitat connectivity (Tournant et al. 2013), this can largely affect the demography of its populations.
Multiple habitat graphs can distinguish several types of totally different habitats and the movements that functionally connect them (i.e., associated with breeding or dispersal). When they are restricted to two habitat types and only consider the links between these distinct habitats, they are a spatial example of a bipartite graph (Haase et al. 2017). However, they can go beyond this simple example and, importantly, can also consider the links among patches of the same type. This more realistic modeling approach is key for revealing the connectivity patterns underpinning landscape complementation processes. This will help detecting how minor land use changes can spark detrimental consequences for the dynamics of subsets of populations, and even put their survival at risk. Note that if the sole path ensuring seasonal migration movements is no longer functional or if the only habitat patch sustaining breeding is destroyed, populations could face extinction on a large scale. The corollary of these intricate dynamics is that depicting them in a more realistic manner also provides insights into which type of habitat should be best restored and, importantly, where. We next present our new modeling approach and its outputs, and how it is seamlessly implemented in the new version of Graphab.
Methods
To construct multiple habitat graphs and benefit from their theoretical advantages described above, we have released a new version of Graphab. This open-source software program makes it possible to build and analyze landscape graphs in a wide range of environments, including a GUI, command-line facilities, a QGIS plugin, and an R package (Foltête et al. 2012, 2021; Savary et al. 2021). The new version, Graphab 3.0, extends previous modeling options to operationalize the multiple habitat graph approach. Creating a project is the starting point of each set of analyses in this software program. At this stage, users can now consider several types of habitats, either by specifying the habitat codes from a categorical raster map of land cover, or by providing a vector map of habitat patches. They can then create sets of least-cost paths according to the cost values they provide to define the resistance surface. The novelty of the new release is that these links can either connect pairs of patches of each habitat type separately, of all habitat types together, or alternatively, can be restricted to cross-connections between habitat types. After specifying topological criteria matching species movement types, landscape graphs are created and can include or not the different types of links created before.
Landscape graphs are easy to visualize using cartographical representations, either directly in Graphab or by exporting outputs to open them in other GIS applications. These graphs are usually analyzed by computing metrics at the graph-level or patch-level. The relevance and potential redundancy of the wide range of existing metrics have been investigated. Most often, a reduced set of metrics representing the amount of reachable habitat at the patch scale (e.g., patch carrying capacity or area), and beyond the patch at a local scale (e.g., local flux metrics) or network scale (e.g., betweenness centrality metric) is sufficient to represent habitat network properties (Rayfield et al. 2011) and explain their effects on biological responses (Mony et al. 2018; Savary et al. 2022; Daniel et al. 2023). We adapted these metrics to multiple habitat graphs. As with single habitat graphs, when links are weighted for computing metrics, every distance between two patches is converted into a dispersal probability by assuming that movement probabilities decay with distance. This is done by using a negative exponential function, as is commonly done in the metapopulation literature (Hanski 1989). Because the multiple habitat graph approach distinguishes several movement types according to the habitat type they connect, the dispersal kernel can vary according to the link types. Second, the connectivity measurement can focus on specific connections between different types of habitats. This amounts to subdividing the overall connectivity into several components. We illustrate this below with both a global metric (i.e., computed at the scale of the entire graph) and a local metric (i.e., computed at the patch scale). Note that we only use two distinct habitat types in the following examples but multiple habitat graphs (and the new version of Graphab) can accommodate more than just two types.
-
The Equivalent Connectivity (EC) metric assesses the amount of reachable habitat at the scale of the entire habitat network. It corresponds to the area of a single patch that will provide species with an equal amount of reachable habitat as the studied habitat patch network, given its number of patches and the resistance of the landscape matrix (Saura et al. 2011). The generic formula is as follows:
$$\begin{aligned} EC = \sqrt{\sum _{i}^{n} \sum _{j}^{n} a_{i} a_{j} e^{-\alpha \times d_{ij}}} \end{aligned}$$(1)The particularity of this computation in multiple habitat graphs is that it can distinguish several components of the overall metric according to the type of patches i and j connected by a link ij. Given two habitat types a and b, we can estimate the contribution of the connections between patches of habitat a alone (\(EC_{aa}\)), of habitat b alone (\(EC_{bb}\)) and of the connections between patches of habitat a and b (\(EC_{ab}\)), such that:
$$\begin{aligned} EC_{all} = \sqrt{EC_{aa}^{2} + EC_{bb}^{2} + EC_{ab}^{2}} \end{aligned}$$(2)Note that for this metric and the following one, the movement path associated with the shortest distance \(d_{ij}\) between two patches of the same type can still cross a patch of another type. We present below how the BC metric can be used to quantify these indirect contributions.
-
The Flux metric estimates the amount of reachable habitat from any given habitat patch. For that purpose, it sums the capacity of other patches weighted by the dispersal probability to these patches, such that:
$$\begin{aligned} F_{i} = \sum _{j}^{n} a_{j} e^{-\alpha \times d_{ij}} \end{aligned}$$(3)with i the index of the focal patch, j the index of the other connected patches among the n habitat patches, \(d_{ij}\) the cost-distance between patches i and j, and \(a_{j}\) the capacity of patch j. Note that the capacity is akin to the patch carrying capacity. Although per default capacities correspond to patch areas, users can specify a custom value which serves as a reliable proxy for the patch demographic potential. In multiple habitat graphs, for a focal patch of habitat type a, F can be computed by considering only the connections to other patches of habitat a (\(F_{aa}\)) or to patches of habitat b (\(F_{ab}\)), and similarly for patches of habitat b (\(F_{bb}\), \(F_{ba}\)).
-
The Betweenness Centrality metric assesses the contribution of a given habitat patch to the connectivity between other patches. It is equal to the number of times the focal patch is located on the least-cost paths on the graph between two other patches, when considering all possible patch pairs and by weighting each pair by the product of their capacities and the dispersal probability between them, such that:
$$\begin{aligned} \begin{array}{l} BC_{i}=\sum _{j} \sum _{k} a_{j} a_{k} e^{-\alpha d_{jk}} \\ j,k \in \left\{ 1, \ldots , n\right\} , k < j, i \in P_{jk} \end{array} \end{aligned}$$(4)with \(P_{jk}\) the set of patch pairs to consider, corresponding to all patch pairs jk (\(i != j, i != k\)) connected by a least-cost path passing through patch i. The multiple habitat graph approach makes it possible to assess the contribution of a patch of habitat a to the connectivity between patches of type a only (\(BC^{a}_{aa}\)), b only (\(BC^{a}_{bb}\)) or between a and b (\(BC^{a}_{ab}\)), and similarly for patches of habitat b (\(BC^{b}_{aa}\), \(BC^{b}_{bb}\), \(BC^{b}_{ab}\)).
The subdivision of metric values into several components described above is also possible when more than two habitat types are distinguished, and for all the other connectivity metrics already included in Graphab. Although not exhaustively described here for the sake of brevity, Graphab 3.0 made most of landscape graph analysis functionalities compatible with the multiple habitat graph approach (e.g., patch addition prioritization, link-level metrics). Similarly, users can identify habitat modules by partitioning the graph or analyze different corridors according to the types of habitats they connect and the movement types they support.
Additionally, to investigate the relationship between landscape graphs and biological data (Foltête et al. 2020), users can include nodes corresponding to their sampling sites in order to compute landscape connectivity metrics at this level with more flexibility. Graphab 3.0 and its user manual for both the GUI and command-line facilities are available at: https://sourcesup.renater.fr/www/graphab/v3/.
Case studies
In the three following case studies, we illustrate how the multiple habitat graph approach contributes to a better understanding of landscape-scale ecological processes and to decision-making in landscape and urban planning. The objective of this section is not to provide a detailed explanation of the data and methods employed in each study case, but rather to provide didactic examples corresponding to potential ecological applications of our framework (see Appendices S1 to S3 for the modeling details).
Considering potential source-sink dynamics in urban biodiversity management
The local biotic and abiotic conditions affecting population eco-evolutionary dynamics in urban habitats are highly influenced by management practices and surrounding urbanization (Des Roches et al. 2021). Despite strong filtering effects reducing taxonomic and functional diversity in cities (Piano et al. 2020), urban habitats can still host a significant part of regional species pools (Aronson et al. 2014). However, the permanence of diversity patterns in these habitats probably depends to a large degree on their connections to peri-urban habitats (Snep et al. 2006; Lepczyk et al. 2017; Stillfried et al. 2017; Wang et al. 2022). Accordingly, the role of habitat connectivity for maintaining biodiversity in cities has given rise to a large body of research (e.g., Beninde et al. (2015); LaPoint et al. (2015); Tannier et al. (2012, 2016); Balbi et al. (2018); Khiali-Miab et al. (2022)), and the development of green infrastructures increasing connectivity is a key objective in urban biodiversity conservation strategies.
(A) Land cover of the study area, located in the Greater Paris, France (48°86’N, 2°72’E). Single habitat graph (B) and multiple habitat graphs (C, D) representing the connectivity of a habitat patch network made of urban patches of wooded habitats (light green) and forests (dark green), both represented in grey in the case of the single habitat graph approach. The connectivity between each patch and the surrounding patches is assessed using the Flux metric (F). Values are displayed with circles at the patch (node) level, with sizes proportional to the metric values. In the single habitat graph (B), these circles are grey and the F metric assesses the amount of both forest and urban wooded habitat reachable from any type of patch. In contrast, in the multiple habitat graph approach, the circles are light green when the F metric assesses the amount of forest reachable from an urban habitat (C) and dark green when it assesses the amount of urban habitat reachable from a forest patch (D). Graph links are uniformly grey in the single habitat graph (B), whereas they take a dark green, light green, or purple color when they depict connections among forests, among urban green spaces, or between forest and urban green spaces, respectively, in multiple habitat graphs (C, D)
Connectivity contrasts revealed by multiple habitat graphs within each habitat type. (a) Relationship between the amount of forest reachable (x-axis) from forest patches (plain circles) or from urban green space patches (triangles), and the amount of urban green spaces (y-axis) reachable from forest or urban green spaces. The amount of reachable habitat is computed with the F metric (see details in main text). Every data point corresponds to a patch. Urban green space patches located on the right side of the vertical dashed line (pale orange) take values of the F metric measuring the connectivity to forest from the 9th decile of its distribution, and are therefore the most connected to forest patches. Similarly, forest patches located above the horizontal dashed line (purple) take values of the F metric measuring the connectivity to urban green spaces from the 9th decile of its distribution, and are therefore the most connected to urban green spaces. (b) Spatial location of the habitat patches according to the relationship between their connectivity to forest patches and urban green spaces. Forest patches above the horizontal dashed line on panel (a) and urban green space patches on the right of the vertical dashed line are represented in the same colors on panel (b)
Determining to which point the biodiversity of urban habitats depends on peri-urban habitats (or conversely) is important for several reasons. First, if population growth rates in urban habitats cannot sustain populations without immigration from peri-urban habitats, these habitats could be considered as sinks (Pulliam 1988; Lepczyk et al. 2017; Stillfried et al. 2017), and increasing their local suitability should then be the priority objective. Second, if movements from peri-urban to urban habitats are more important than movements between urban habitats for population dynamics and diversity patterns, connectivity conservation measures should target this type of movements. Third, these potential source-sink effects could also affect the ability of populations to adapt to urban environments (Szulkin et al. 2020; Verrelli et al. 2022). Finally, in certain contexts, urban habitat patches have been shown to act as refuges for various species (Baldock et al. 2015; Ives et al. 2016). Such a phenomenon is expected when surrounding landscapes have been subject to substantial anthropogenic disturbances, and potentially turns urban populations into source populations for peri-urban populations.
To detect and map these dynamics towards guiding urban biodiversity conservation, it is therefore essential to consider the spatial heterogeneity of habitat types in cities and their surroundings. This heterogeneity does not reside on the fact that the species of interest use habitats that modelers commonly assign to different categories, or move between them at specific periods. Instead, the species of interest use structurally similar habitats (e.g., wooded urban parks and peri-urban forests, or private flowered lawns and grasslands) that are located in areas reshaped by humans to varying degrees, managed differently, and therefore varying in quality. Multiple habitat graphs can differentiate urban and peri-urban habitats, enabling modelers to assess the individual contribution of each habitat type to the overall habitat connectivity. It also allows for the identification of urban patches that contribute most to the connections with peri-urban habitats, and conversely, the peri-urban patches that facilitate movements to urban habitats.
We illustrate this approach by modeling the network of forest patches and urban green spaces in a highly urbanized area in the Greater Paris, France (48°86’N, 2°72’E; Fig. 2; and see Appendix S1 for further modeling details). We used a 2017 land cover map provided by Institut Paris Région, simplified to include 9 different land cover types and rasterized at a resolution of 2 m. Least-cost paths were computed between pairs of habitat patches, irrespective of their nature, following a minimum planar graph topology (see cost scenario in Appendix S1: Tables S1 and S2). On the obtained graph, all types of potential links were conserved such that two forest patches, two urban green spaces or one patch of each type could be connected. We then computed the Flux (F) metric at the patch level, and could thereby assess the connectivity of forest patches to urban green spaces or to other forest patches, and conversely. This analysis revealed the complementarity of forest and urban green space patches in this area for the movements and dynamics of forest species surviving urban conditions (Figure 2). Moreover, Fig. 3 reveals the better resolution gained in the analysis by using multiple habitat graphs instead of single habitat graphs. Indeed, the new approach allows for patch prioritization at the habitat type level, while considering the connection among habitat types. This reveals both the forest patches most connected to urban green spaces and the urban green spaces that are the most connected to forest patches. Because these two sets of patches do not necessarily coincide in an urban context (Fig. 3), these results could help managers identify the most important patches, both within and outside the urban fabric. These analyses could also provide relevant information for sampling populations when investigating urban adaptations and how gene flow moderates it.
Improving semi-natural area management as part of conservation biological control strategies
Our proposed model could also find applications when modeling ecological processes in agroecosystems. Indeed, disentangling trophic interactions and their consequences for population dynamics in agroecosystems is critical for ensuring sustainable crop production (Benton et al. 2003). Although pest populations have been increasingly regulated using pesticides in the past century, conservation biological control strategies can be implemented to prevent pest outbreaks (Tscharntke et al. 2007), either by managing their resources (e.g., with frequent crop rotations) or by favoring natural enemies (e.g., entomophagous arthropods; Gurr et al. (2017)). The success of the latter top-down strategy has been shown empirically (e.g., Woodcock et al. (2016); Aguilera et al. (2020)). It depends on landscape supplementation processes and is contingent upon natural enemy movements between semi-natural habitats and crop fields (Rand et al. 2006; Tscharntke et al. 2012). This stems from the fact that natural enemy species such as carabid species or other beetles often breed or overwinter in field margins or fallow land and then spill-over into arable crops to feed on pest species (Schellhorn et al. 2014). These processes have been studied for a long time (Tscharntke et al. 2005; Rand et al. 2006), and have justified subsidies for agri-environmental measures as part of the European Common Agricultural Policy (e.g., beetle banks, flower strips).
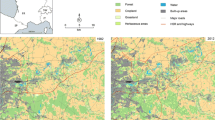
Connectivity of the network of annual crops and semi-natural habitats in a French agricultural landscape (48°08’N, 1°40’E), as modeled using the multiple habitat graph approach for the years 2010 and 2020. Annual crops (orange in A) include cereals, maize, beets, potatoes, oil-protein crops and other annual cash crops, and exclude grasslands, perennial fodder crops or plantations. Semi-natural patches (yellow in A) include field margins, fallow land, and grassy strips. The left column corresponds to the year 2010, and the right column to 2020. In the bottom panels representing multiple habitat graphs (B), the pruned links of the graph are represented in purple. The circles representing each node have a size proportional to the values of the F metric, assessing the amount of crop field habitat reachable from semi-natural patches (yellow), or of semi-natural habitat reachable from crop fields (orange)
Notwithstanding their alleged advantages, some studies did not show evidence of the benefits of semi-natural areas for biocontrol (Veres et al. 2013; Duflot et al. 2015) and several explanations can be given. First, the legacy of intensive pesticide use or past extinctions of natural enemy species can explain why conserving semi-natural areas in the landscape falls short in favoring biocontrol. Apart from these mechanistic explanations, the way we account for landscape composition and configuration when testing for the influence of semi-natural habitats on biocontrol can also be brought into question (Veres et al. 2013; Martin et al. 2019). On the one hand, some types of semi-natural habitats can either favor or limit the presence of pest species, as for instance woody habitats (e.g., woodland and hedgerows) and open habitats (e.g., grassy strips or fallow land), respectively (Tougeron et al. 2022). On the other hand, landscape configuration may moderate the presence of natural enemies in crop fields (Perović et al. 2015), as the core of fields can be too far from semi-natural habitats to be reached or because some woody habitats are barriers for some flying arthropods.
In light of these aforementioned elements, we need to better account for the spatial heterogeneity and connectivity of habitats in agricultural landscapes if we are to shed light on biocontrol drivers, guide agricultural subsidy policies and farmer decision-making (Batáry et al. 2011; Veres et al. 2013). Multiple habitat graphs may prove helpful in that respect because they can model natural enemy movements between semi-natural habitats and crop fields in a spatially-explicit way. The habitat network would then consist of distinct types of nodes, representing either crop fields or semi-natural open habitats. The set of links will only connect different types of nodes, in order to explicitly model landscape supplementation processes sustaining biocontrol while potentially accounting for variations in the matrix permeability to movements linked to its heterogeneity (e.g. hedgerows, thickets, roads). One can then assess the overall connectivity of the network at the landscape or farm level using global connectivity metrics. These metrics can be used to test the influence of semi-natural habitats on biocontrol when data on natural enemies, pest populations, or crop damages are available at the landscape- or farm-level. Note that when data are available for several years, the influence of crop rotations on biocontrol and pest outbreak dynamics can also be assessed. Besides, metrics computed at the local-level, i.e., at the level of individual crop fields or semi-natural habitat patches, can reveal which fields are potentially more reachable by natural enemies, and which field margins, fallow land, or grassy strips could favor most biocontrol. This information will give some leeway to improve parcel configurations towards maximizing local or farm-level biocontrol.
To provide an example of this modeling approach (Fig. 4), we modeled the network of annual crops and semi-natural habitats in a French agricultural landscape (48°08’N, 1°40’E) for the years 2010 and 2020 (see Appendix S2 for further modeling details). We used the national agricultural parcel database (Registre Parcellaire Graphique, ASP) to identify two types of habitat patches: (i) annual crops, including cereals, maize, beets, potatoes, oil-protein crops and other annual cash crops, and excluding grasslands, perennial fodder crops or plantations, and (ii) field margins, fallow land, and grassy strips. We then computed the movement paths between crops and semi-natural habitats, excluding paths between habitats of the same type. We assumed that Euclidean distances reflected the cost of dispersal across mostly open areas of entomophagous arthropods regulating pest species such as aphids or butterflies. After building the two multiple habitat graphs (one for each year), we computed the Equivalent Connectivity (EC) metric at the graph level and the Flux metric (F) at the patch level for both years. This diachronic analysis revealed substantial changes from 2010 to 2020. First, the number of semi-natural habitat patches has increased nine-fold in 10 years, while their total area has increased by only 57 %. In contrast, the number of crop fields has increased by 26 % for an overall decrease in total area of 6 %. From 2010 to 2020, these contrasting changes jointly affecting the number, mean size, and total areas of crop fields and semi-natural patches translated into an increase by 28 % of the connectivity of the network they form. These changes in crop rotations and semi-natural area management are visible on the map, which sketches where local gains (or losses) of connectivity have occurred (Fig. 4). Local metric values could be used to understand pest outbreaks, assess the performance of farming strategies, and help designing crop rotations maximizing the potential for biological control over time. They also reveal the consequences of agricultural policies implemented over the last decades in Europe, which have significantly increased the amount of semi-natural areas and modified crop planning in the study area.
Accounting for seasonal migrations for conserving amphibian composite habitat networks
Ecological processes and the movements they cause often go beyond the limits of a single habitat type. Some species use different types of habitats during their life cycle (e.g., amphibians, bats, fish) and are therefore particularly sensitive to the spatial arrangement of these different types of habitats and their connectivity. Amphibians are an example of biphasic species, breeding in aquatic areas (e.g., ponds), but spending the rest of their life cycles in terrestrial habitats. This life history strategy leaves a typical imprint on their movement patterns, which deeply vary over spatial and temporal scales (Cayuela et al. 2020). Individuals move between aquatic habitats and more or less distant terrestrial habitats during seasonal migrations, but also between two aquatic habitats via one or more intermediate terrestrial habitats during inter-annual dispersal.
Despite the peculiar pattern of these different types of movements, many amphibian conservation studies are focused on a single ecological process (mostly dispersal) and evaluate connectivity between breeding habitats (e.g., Clauzel et al. (2013)). However, focusing solely on one type of movement overlooks an integral aspect of their life cycle, namely seasonal migration, which plays a crucial role in maintaining the viability of amphibian populations (Bailey and Muths 2019). Furthermore, assessing connectivity at the dispersal scale by focusing on direct connections between breeding habitats does not match the actual movement behavior of amphibians. The latter is best modeled by assuming that dispersal connections among breeding ponds involve an intermediate step through a terrestrial habitat used during and outside breeding periods. Yet, similar assumptions have only been considered in amphibian connectivity models based on highly parameterized individual-based approaches (see the study by Mims et al. (2023)).
In that context, multiple habitat graphs overcome the limitations of classical single habitat approaches. The graph created to illustrate the application of our approach to this biological model (Fig. 5) includes two distinct habitat types (aquatic and terrestrial habitats) linked by a set of inter-habitat links. This model allows for quantitative and cartographic connectivity assessments that are specifically tailored to the ecological process of interest. Amphibian migration will be mostly affected by connectivity patterns driving fine-scale movements between aquatic and terrestrial habitats. In contrast, amphibian dispersal and gene flow will depend on larger-scale indirect movements between aquatic habitats stepping through terrestrial habitats. In that context, connectivity metrics computed assuming different connections and movement distances will be relevant predictors of different biological patterns. For instance, the Flux metric (F) parameterized for movement distances commonly covered during seasonal migrations assesses the amount of aquatic habitat reachable from a patch of terrestrial habitat, and vice versa. It therefore reflects the contribution of these habitat patches to annual population dynamics. In contrast, the same metric computed by assuming a movement kernel characterizing the behavior of dispersing individuals evaluates the potential gene flow between breeding habitats over multiple years. Note that in that case, the philopatry degree of breeders will determine the frequency and spatial distribution of gene flow events per breeding migration event (see Cayuela et al. (2020), for an empirical evidence). Finally, the topology of inter-habitat links provides an advantage over single habitat graphs to realistically compute centrality metrics such as the Betweenness Centrality Index. This index can assess the contribution of each terrestrial habitat (or each link) to multi-generational dispersal movements among all breeding habitat pairs, thereby revealing the backbone of gene flow events (Estrada and Bodin 2008; Urban et al. 2009). From a conservation point of view, mapping the most important migration paths can lead to targeted measures for reducing amphibians’ road kills, while identifying gene flow patterns can help sustaining genetic diversity for long-term adaptation potential. Both endeavors are equally important for a taxon facing high extinction risks due to historical hydrologic management, climate change, and wildlife disease, among others (Díaz et al. 2019).
To provide an example, we modeled the network of amphibian species in a mainly agricultural area in the south of Paris (48°44’N, 2°17’E, Clauzel et al. (2024); see Appendix S3 for further modeling details). We built a land cover map for the year 2021 by combining land cover data from Institut Paris Région, the locations of the main linear transport infrastructures provided by the National Institute of Geographic and Forest Information (IGN), and a pond inventory from the SNPN naturalist association. The land cover map includes 21 different land cover types at a spatial resolution of 3 m. We compared the network structure and connectivity values (Flux metric) at the dispersal scale between a single habitat graph (Fig. 5A) and a multiple habitat graph (Fig. 5B).
Connectivity of an amphibian ecological network in a French agricultural landscape (48°44’N, 2°17’E), modeled using a single habitat (A) and a multiple habitat graph approach (B). Whereas the single habitat graph contains only aquatic habitats, the multiple habitat graph contains aquatic habitats (blue) and terrestrial habitats (purple) connected by an inter-habitat link set. In both cases, the link topology is complete. The connectivity between each patch and its neighboring patches was assessed using the Flux metric (F). We assumed that the movement distances used for the computation match typical dispersal distances for aquatic habitats (A, B). Values are displayed with circles at the patch (node) level, with sizes proportional to the metric values. These circles are blue when the F metric assesses the amount of aquatic habitats reachable from another aquatic habitat patch by direct connections (A) or through indirect connections via an intermediate terrestrial habitat patch (B)
This analysis revealed some differences in network structure. Some aquatic habitats (e.g., in the North) appeared to be rather isolated in the single habitat graph but were connected to other habitats via terrestrial habitats in the multiple habitat graph, although in a fragile way. The latter model thus highlighted the key role of terrestrial habitats for both migration and dispersal. When the local aquatic habitat connectivity was calculated at the dispersal scale, the Flux metric took similar values in both approaches. This suggests that the classical single habitat approach could be sufficient for an assessment of aquatic habitat connectivity. In contrast, the multiple habitat approach would be of greater interest for detailed and multi-scale connectivity analyses, making it possible to break down the modeling outputs reflecting either migration or dispersal processes. Accordingly, this approach can be particularly useful for habitat restoration. Clauzel et al. (2024) showed that improving connectivity sometimes requires the creation of a terrestrial habitat associated with an aquatic habitat. The efficiency of such a strategy is conditional upon a proper consideration of patch types and locations within ecological networks at the landscape scale. In that context, the approach we introduce will prove helpful for landscape managers to design conservation or restoration actions. Multiple habitat graphs can also be used to assess the effects of landscape transformations (e.g. conversion of grasslands to crops, urbanization, restoration of ponds, reforestation) affecting the different types of habitats used by amphibian species, thus revealing which ecological process would be most affected.
Perspectives for future applications and improvements
By focusing on a single type of habitat and movement, common connectivity models fall short in depicting how habitat connectivity gives rise to landscape-scale ecological processes driving population dynamics in significant ways. To bridge the gaps between landscape ecology theory and modeling approaches, and hopefully between models and reality, we propose a new version of the Graphab software program that operationalizes the concept of multiple habitat graphs. Although we described several examples in which these tools would be relevant for research and conservation applications in various landscape contexts, this new approach opens avenues for other applications and further extensions.
Landscape graph connectivity modeling is most relevant for a given species or a set of specialist species occupying the same type of discrete habitat patches (Galpern et al. 2011). However, by considering different types of habitats in the same model, our approach relaxes this constraint and offers the possibility to model simultaneously the connectivity for species having a wide range of ecological requirements, coexisting at landscape scales in partially overlapping habitats, and potentially sharing movement patterns. This could help explain how landscape structure drives the specialization degree of communities (Lami et al. 2021). Similarly, multiple habitat graphs can improve predictions in spatial epidemiology by modeling the interaction of species occupying the same type of habitat during their encounter period, but different habitat types most of the time (Gilbertson et al. 2023). For example, the spread of pathogens responsible for Lyme disease or echinococcosis depends on the movements of rodent and carnivorous mammal species potentially occupying different types of habitats (Giraudoux et al. 2003; Ostfeld et al. 2005; Raoul et al. 2015).
Besides, while connectivity conservation strategies often include several types of habitats (e.g., Mimet et al. (2013); Oehri et al. (2023)), the joint consideration of their connectivity is still lagging behind. Environmental management schemes based on ecological networks (e.g., green and blue infrastructures) tend to compartmentalize conservation issues by analyzing each habitat network independently. This partly stems from the current lack of methods and tools available to landscape managers for combining different networks and detecting areas with multiple-habitat connectivity issues (Savary et al. 2024). Multiple habitat graphs can also help analyze the respective contributions to connectivity of habitat types defined using administrative criteria, such as protected areas (Santini et al. 2016), private lands (Bargelt et al. 2020), or retention ponds (Clevenot et al. 2022).
While our approach allows for the consideration of multiple habitat types in connectivity models, it does not tackle the challenge of identifying these habitat types based on their ecological functions, such as being a source, sink, supplementing, or complementing habitat. If the main objective justifying connectivity analyses is to assess the respective contributions of areas that share comparable structural and environmental conditions but that differ in terms of management, conservation status, or ownership, the definition and delineation of habitat types can be arbitrary and based on these human-defined distinctions. Nevertheless, ecological differences among habitat types can be informed by expert knowledge, as for instance in our case studies. It is common practice when implementing the single habitat graph approach to select habitat patches from the output of species distribution models (Foltête et al. 2020). However, these models rarely consider the fact that species use different habitat and resource throughout the year. As a consequence, although this approach provided an empirically grounded solution to the identification of habitat patches for this approach, this workaround might not fit the multiple habitat graph approach as adequately. The usefulness of any ecological model highly depends on the relevance of its parameters to the pursued objectives. Because our modeling approach adds complexity to common modeling approaches, this novelty must go hand in hand with the use of advanced empirical methods and expert knowledge for defining multiple habitats in a realistic way. Resource Selection or Utilization Functions fitted from telemetry data at different life cycle stages could, for instance, provide key insights into the seasonal use of habitats and resources by animals (e.g., Boggie et al. (2017); Aiello et al. (2023)).
Finally, in the new release of the Graphab software, we have adapted commonly used graph-based connectivity metrics to multiple habitat graphs. Nonetheless, new metrics directly inspired from this type of graph could further extend the toolbox of connectivity modeling. Besides, we did not present the use of modularity analyses considering how subsets of habitat patches of different types can ensure the optimal accomplishment of the different stages in species life cycles. We believe these aspects represent promising research avenues for dedicated landscape ecologists and conservation specialists.
References
Aguilera G, Roslin T, Miller K, Tamburini G, Birkhofer K, Caballero-Lopez B, Ann-Marie Lindström Sandra, Erik Ökinger, Maj Rundlöf, Adrien Rusch, Smith Henrik G, Bommarco R (2020) Crop diversity benefits carabid and pollinator communities in landscapes with semi-natural habitats. J Appl Ecol 57(11):2170–2179.
Aiello CM, Galloway NL, Prentice PR, Darby NW, Hughson D, Epps CW (2023) Movement models and simulation reveal highway impacts and mitigation opportunities for a metapopulation-distributed species. Landsc Ecol 38(4):1085–1103.
Aronson MF, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NS, Cilliers S, Clarkson B, Dobbs C (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc Royal Soc B: Biol Sci 281(1780):20133330.
Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev 88(2):310–326
Bailey LL, Muths E (2019) Integrating amphibian movement studies across scales better informs conservation decisions. Biol Conserv 236:261–268
Balbi M, Ernoult A, Poli P, Madec L, Guiller A, Martin M-C, Nabucet Jean, Beaujouan Véronique, Petit EJ (2018) Functional connectivity in replicated urban landscapes in the land snail (Cornu aspersum). Mol Ecol 27(6):1357–1370
Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Osgathorpe LM, Potts Simon G, Robertson Kirsty M, Scott Anna V, Stone Graham N, Vaughan Ian P, Memmott J (2015) Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc Royal Soc B: Biol Sci 282(1803):20142849.
Bargelt L, Fortin M-J, Murray DL (2020) Assessing connectivity and the contribution of private lands to protected area networks in the united states. PLoS One 15(3):e0228946
Batáry P, Fischer J, Báldi A, Crist TO, Tscharntke T (2011) Does habitat heterogeneity increase farmland biodiversity? Front Ecol Environ 9(3):152–153
Beninde J, Veith M, Hochkirch A (2015) Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecol lett 18(6):581–592
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecol Evol 18(4):182–188
Boggie MA, Strong CR, Lusk D, Carleton SA, Gould WR, Howard RL, Nichols Clay, Falkowski Michael, Hagen C (2017) Impacts of Mesquite Distribution on Seasonal Space Use of Lesser Prairie-Chickens. Rangel Ecol Manag 70(1):68–77.
Borthagaray AI, Barreneche JM, Abades S, Arim M (2014) Modularity along organism dispersal gradients challenges a prevailing view of abrupt transitions in animal landscape perception. Ecography 37(6):564–571
Cayuela H, Besnard A, Cote J, Laporte M, Bonnaire E, Pichenot J, Schtickzelle Nicolas, Bellec Arnaud, Joly Pierre, Léna J-P (2020) Anthropogenic disturbance drives dispersal syndromes, demography, and gene flow in amphibian populations. Ecol Monogr 90(2):e01406
Cayuela H, Valenzuela-Sánchez A, Teulier L, Martínez-Solano Í, Léna J-P, Merilä J, Muths Erin, Shine Richard, Quay Ludivine, Denoël Mathieu et al (2020) Determinants and consequences of dispersal in vertebrates with complex life cycles: a review of pond-breeding amphibians. The Q Rev Biol 95(1):1–36
Clauzel C, Girardet X, Foltêete J-C (2013) Impact assessment of a high-speed railway line on species distribution: Application to the European tree frog (Hyla arborea) in Franche-Comté. J Environ Manag 127:125–134
Clauzel C, Godet C, Tarabon S, Eggert C, Vuidel G, Bailleul M, Miaud C (2024) From single to multiple habitat connectivity: The key role of composite ecological networks for amphibian conservation and habitat restoration. Biol Conserv 289:110418.
Clevenot L, Clauzel C, Tourret K, Carre C, Pech P (2022) How much can highway stormwater ponds contribute to amphibian ecological network connectivity? Impact Assess Proj Apprais 40(6):517–530
Crooks, K.R., & Sanjayan, M. (2006). Connectivity conservation (Vol. 14). Cambridge University Press
Daniel A, Savary P, Foltêete J-C, Khimoun A, Faivre B, Ollivier A, Éraud Cyril, Moal Hervé, Vuidel Gilles, Garnier S (2023) Validating graph-based connectivity models with independent presence-absence and genetic data sets. Conserv Biol 37(3):e14047
Den Boer PJ (1968) Spreading of risk and stabilization of animal numbers. Acta Biotheor 18(1–4):165–194
Des Roches S, Brans KI, Lambert MR, Rivkin LR, Savage AM, Schell CJ, Correa Cristian, Meester De, Luc Diamond, Sarah E, Grimm Nancy B et al (2021) Socio-eco-evolutionary dynamics in cities. Evol Appl 14(1):248–267
Díaz, S.M., Settele, J., Brondízio, E., Ngo, H., Guèze, M., Agard, J., Arneth, Almut, Balvanera, Patricia, Brauman, Kate, Butchart, Stuart, others (2019). The global assessment report on biodiversity and ecosystem services: Summary for policy makers (Tech. Rep.). Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services
Duflot R, Aviron S, Ernoult A, Fahrig L, Burel F (2015) Reconsidering the role of ‘semi-natural habitat’ in agricultural landscape biodiversity: a case study. Ecol Res 30(1):75–83.
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65(1):169–175
Estrada E, Bodin Ö (2008) Using network centrality measures to manage landscape connectivity. Ecol Appl 18(7):1810–1825.
Foltêete J-C, Clauzel C, Vuidel G (2012) A software tool dedicated to the modelling of landscape networks. Environ Modelling Softw 38:316–327
Foltêete J-C, Savary P, Clauzel C, Bourgeois M, Girardet X, Sahraoui Y, Vuidel Gilles, Garnier S (2020) Coupling landscape graph modeling and biological data: a review. Landsc Ecol 35(5):1035–1052
Foltêete J-C, Vuidel G, Savary P, Clauzel C, Sahraoui Y, Girardet X, Bourgeois M (2021) Graphab: an application for modeling and managing ecological habitat networks. Softw Impacts 8:100065
Forman RT (1995) Some general principles of landscape and regional ecology. Landsc Ecol 10(3):133–142
Fortin M-J, Dale MR, Brimacombe C (2021) Network ecology in dynamic landscapes. Proc Royal Soc B 288(1949):20201889
Galpern P, Manseau M, Fall A (2011) Patch-based graphs of landscape connectivity: a guide to construction, analysis and application for conservation. Biol Conserv 144(1):44–55
Gilbertson MLJ, Hart SN, VanderWaal K, Onorato D, Cunningham M, VandeWoude S, Craft ME (2023) Seasonal changes in network connectivity and consequences for pathogen transmission in a solitary carnivore. Sci Rep 13(1):17802.
Giraudoux P, Craig PS, Delattre P, Bao G, Bartholomot B, Harraga S, Quéré J-P, Raoul F, Wang Y, Shi D, Vuitton D-A (2003) Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology 127(1):121–131.
Gonzalez A, Thompson P, Loreau M (2017) Spatial ecological networks: planning for sustainability in the long-term. Current Opin Environ Sustain 29:187–197
Gurr GM, Wratten SD, Landis DA, You M (2017) Habitat management to suppress pest populations: progress and prospects. Annual Rev Entomol 62:91–109
Haase CG, Fletcher RJ, Slone DH, Reid JP, Butler SM (2017) Landscape complementation revealed through bipartite networks: an example with the Florida manatee. Landsc Ecol 32(10):1999–2014.
Hanski I (1989) Metapopulation dynamics: does it help to have more of the same? Trends Ecol Evol 4(4):113–114
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy Sarah A, Fuller Richard A, Laura Mumaw, Laura Rayner, Ross Rowe, Valentine Leonie E, Kendal D (2016) Cities are hotspots for threatened species. Glob Ecol Biogeogr 25(1):117–126.
Keitt T, Urban D, Milne B (1997) Detecting critical scales in fragmented landscapes. Conserv Ecol 1(1):1–17
Khiali-Miab A, Grêet-Regamey A, Axhausen KW, van Strien MJ (2022) A network optimisation approach to identify trade-offs between socio-economic and ecological objectives for regional integrated planning. City Environ Interact 13:100078
Lami F, Bartomeus I, Nardi D, Beduschi T, Boscutti F, Pantini P, Marini L (2021) Species-habitat networks elucidate landscape effects on habitat specialisation of natural enemies and pollinators. Ecol Lett 24(2):288–297.
LaPoint S, Balkenhol N, Hale J, Sadler J, van der Ree R (2015) Ecological connectivity research in urban areas. Funct Ecol 29(7):868–878
Lepczyk CA, Aronson MF, Evans KL, Goddard MA, Lerman SB, MacIvor JS (2017) Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 67(9):799–807
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Am Entomol 15(3):237–240
Levins R (1970) Extinction. Some mathematical questions in biology
Loreau M, Daufresne T, Gonzalez A, Gravel D, Guichard F, Leroux SJ, Mouquet N (2013) Unifying sources and sinks in ecology and Earth sciences. Biol Rev 88(2):365–379.
MacArthur R, Wilson E (1967) Theor Island Biogeogr. Princeton University Press, Princeton, NJ
Martensen AC, Saura S, Fortin M-J (2017) Spatio-temporal connectivity: assessing the amount of reachable habitat in dynamic landscapes. Methods Ecol Evol 8(10):1253–1264
Martin EA, Dainese M, Clough Y, Báldi A, Bommarco R, Gagic V, Steffan-Dewenter I (2019) The interplay of landscape composition and configuration: new pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol Lett 22(7):1083–1094.
McRae BH (2006) Isolation by resistance. Evolution 60(8):1551–1561
Mimet A, Houet T, Julliard R, Simon L (2013) Assessing functional connectivity: a landscape approach for handling multiple ecological requirements. Methods Ecol Evol 4(5):453–463
Mims MC, Drake JC, Lawler JJ, Olden JD (2023) Simulating the response of a threatened amphibian to climate-induced reductions in breeding habitat. Landsc Ecol 38(4):1051–1068.
Mony C, Abadie J, Gil-Tena A, Burel F, Ernoult A (2018) Effects of connectivity on animal-dispersed forest plant communities in agriculture-dominated landscapes. J Veg Sci 29(2):167–178
Mouquet N, Loreau M (2003) Community patterns in source-sink metacommunities. Am Nat 162(5):544–557
Nicoletti G, Padmanabha P, Azaele S, Suweis S, Rinaldo A, Maritan A (2023) Emergent encoding of dispersal network topologies in spatial metapopulation models. Proc Nat Acad Sci 120(46):e2311548120
Oehri J, Wood SLR, Touratier E, Leung B, Gonzalez A (2023) Rapid evaluation of habitat connectivity change to safeguard multispecies persistence in human-transformed landscapes. bioRxiv. https://doi.org/10.1101/2023.11.23.568419
Ostfeld RS, Glass GE, Keesing F (2005) Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol 20(6):328–336
Perović D, Gámez-Virués S, Börschig C, Klein A-M, Krauss J, Steckel J, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52(2):505–513.
Piano E, Souffreau C, Merckx T, Baardsen LF, Backeljau T, Bonte D, Hendrickx F (2020) Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Global Change Biol 26(3):1196–1211.
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132(5):652–661
Rand TA, Tylianakis JM, Tscharntke T (2006) Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett 9(5):603–614
Raoul F, Hegglin D, Giraudoux P (2015) Trophic ecology, behaviour and host population dynamics in Echinococcus multilocularis transmission. Vet Parasitol 213(3):162–171.
Rayfield B, Fortin M-J, Fall A (2011) Connectivity for conservation: a framework to classify network measures. Ecology 92(4):847–858
Resasco J (2019) Meta-analysis on a decade of testing corridor efficacy: what new have we learned? Current Landsc Ecol Rep 4(3):61–69
Santini L, Saura S, Rondinini C (2016) Connectivity of the global network of protected areas. Diver Distrib 22(2):199–211
Saura S, Estreguil C, Mouton C, Rodríguez-Freire M (2011) Network analysis to assess landscape connectivity trends: application to European forests (1990–2000). Ecol Indic 11(2):407–416
Savary P, Foltêete J-C, Moal H, Vuidel G, Garnier S (2021) graph4lg: a package for constructing and analysing graphs for landscape genetics in R. Methods Ecol Evol 12(3):539–547
Savary P, Foltêete J-C, van Strien MJ, Moal H, Vuidel G, Garnier S (2022) Assessing the influence of the amount of reachable habitat on genetic structure using landscape and genetic graphs. Heredity 128(2):120–131
Savary, P., Lessard, J.-P., Peres-Neto, P.R. (2024). Heterogeneous dispersal networks to improve biodiversity science. Trends in Ecology & Evolution, 39 (3)
Schellhorn N, Bianchi F, Hsu C (2014) Movement of entomophagous arthropods in agricultural landscapes: links to pest suppression. Annual Rev Entomol 59:559–581
Schlägel UE, Grimm V, Blaum N, Colangeli P, Dammhahn M, Eccard JA et al (2020) Movement-mediated community assembly and coexistence. Biol Rev 95(4):1073–1096
Snep R, Opdam P, Baveco J, WallisDeVries M, Timmermans W, Kwak R, Kuypers V (2006) How peri-urban areas can strengthen animal populations within cities: A modeling approach. Biol Conserv 127(3):345–355
Stillfried M, Fickel J, Börner K, Wittstatt U, Heddergott M, Ortmann S, Frantz AC (2017) Do cities represent sources, sinks or isolated islands for urban wild boar population structure? J Appl Ecol 54(1):272–281
Szulkin M, Munshi-South J, Charmantier A (2020) Urban Evolu Biol. Oxford University Press, USA
Tannier C, Bourgeois M, Houot H, Foltêete J-C (2016) Impact of urban developments on the functional connectivity of forested habitats: a joint contribution of advanced urban models and landscape graphs. Land Use Policy 52:76–91
Tannier C, Foltêete J-C, Girardet X (2012) Assessing the capacity of different urban forms to preserve the connectivity of ecological habitats. Landsc Urb Plan 105(1):128–139
Taylor PD, Fahrig L, Henein K, Merriam G (1993) Connectivity is a vital element of landscape structure. Oikos 68(3):571–573
Taylor, P.D., Fahrig, L., With, K.A. (2006). Landscape connectivity: a return to the basics. K.R. Crooks & M. Sanjayan (Eds.), Connectivity conservation (pp. 29–43). Cambridge University Press
Tougeron K, Couthouis E, Marrec R, Barascou L, Baudry J, Boussard H, van Baaren J (2022) Multi-scale approach to biodiversity proxies of biological control service in European farmlands. Sci Total Environ 822:153569.
Tournant P, Afonso E, Rouée S, Giraudoux P, Foltêete J-C (2013) Evaluating the effect of habitat connectivity on the distribution of lesser horseshoe bat maternity roosts using landscape graphs. Biol Conserv 164:39–49
Tscharntke T, Bommarco R, Clough Y, Crist TO, Kleijn D, Rand TA, Vidal S (2007) Conservation biological control and enemy diversity on a landscape scale. Biol Control 43(3):294–309.
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol Lett 8(8):857–874
Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L, Batáry P, Westphal C (2012) Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol Rev 87(3):661–685.
Turner MG (1989) Landscape ecology - the effect of pattern on process. Annual Rev Ecol Syst 20(1):171–197
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82(5):1205–1218
Urban DL, Minor ES, Treml EA, Schick RS (2009) Graph models of habitat mosaics. Ecol Lett 12(3):260–273.
Uroy L, Alignier A, Mony C, Foltêete J-C, Ernoult A (2021) How to assess the temporal dynamics of landscape connectivity in ever-changing landscapes: a literature review. Landsc Ecol 36:2487–2504
Veres A, Petit S, Conord C, Lavigne C (2013) Does landscape composition affect pest abundance and their control by natural enemies? a review. Agric, Ecosyst Environ 166:110–117
Verrelli BC, Alberti M, Des Roches S, Harris NC, Hendry AP, Johnson MT et al (2022) A global horizon scan for urban evolutionary ecology. Trends Ecol Evol 37(11):1006–1019
Wang R, Zhu Q-C, Zhang Y-Y, Chen X-Y (2022) Biodiversity at disequilibrium: updating conservation strategies in cities. Trends Ecol Evol 37(3):193–196
Whitcomb B, Whitcomb R, Bystrak D (1977) Island biogeography and ‘habitat islands’ of eastern forest. iii. long-term turnover and effects of selective logging on the avifauna of forest fragments. Am Birds 31(1):17–23
Woodcock BA, Bullock JM, McCracken M, Chapman RE, Ball SL, Edwards ME, Pywell RF (2016) Spill-over of pest control and pollination services into arable crops. Agric, Ecosyst Environ 231:15–23
Acknowledgements
We are very grateful to Marc Barra from the Agence Régionale de la Biodiversité Île-de-France that provided land cover data for the case study. Some computations were performed on the supercomputer facilities of the Mésocentre de calcul de Franche-Comté. PS is funded by a Concordia Horizon postdoctoral fellowship and the Canada Research Chair in Spatial Ecology and Biodiversity. The part of the work on multiple graphs for amphibians was financed by the French Foundation for Biodiversity Research, the French Ministry of Ecological Transition and the French Biodiversity Office (INTERFACE project). We thank Simon Tarabon, Claire Godet, Christophe Eggert, and Claude Miaud for their support on the multiple habitat approach for amphibians.
Author information
Authors and Affiliations
Contributions
All authors contributed to the initial ideas of the project. GV developed the new version of the Graphab software program and updated users’ manuals. PS and CC wrote the initial draft of the manuscript, which was significantly edited by all authors. GV, CC and PS carried out the case study analyses. XG made the figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary information
Supplementary information are provided in a supplementary file and data are available in Zenodo at {https://doi.org/10.5281/zenodo.10576225}. (pdf 339KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Savary, P., Clauzel, C., Foltête, JC. et al. Multiple habitat graphs: how connectivity brings forth landscape ecological processes. Landsc Ecol 39, 168 (2024). https://doi.org/10.1007/s10980-024-01947-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01947-4