Abstract
Context
Species distribution models (SDMs) may provide accurate predictions of species occurrence across space and time, being critical for effective conservation planning.
Objectives
Focusing on the little bustard (Tetrax tetrax), an endangered grassland bird, we aimed to: (i) characterise the drivers of the species distribution along its key phenological phases (winter, breeding, and post-breeding); and (ii) quantify spatio-temporal variation in habitat suitability across phenological phases and over the years 2005–2021.
Methods
Combining remotely sensed metrics at high temporal resolution (MODIS) with long-term (> 12 years) GPS telemetry data collected for 91 individuals at one of the species’ main strongholds within the Iberian Peninsula, we built SDMs (250 m resolution) for the species key phenological phases.
Results
The use of both dynamic and static predictors unveiled previously unknown ecological responses by little bustards, revealing a marked change in the spatial distribution of suitable habitat among phenological phases. Long-term habitat suitability trends showed considerable fluctuations, mainly in the breeding and post-breeding phases. Overall, SDM projections into the past revealed that while the species’ winter and post-breeding habitats apparently increased since 2005, suitable habitat during the species’ most critical phenological phase, breeding, apparently reduced in area over time.
Conclusions
Our findings show that matching remotely sensed data with GPS tracking data results in accurate habitat suitability predictions throughout the yearly cycle. Additionally, our findings stress the importance of quantifying habitat loss and its potential impact on little bustard decline over nearly 20 years. Spatio-temporal variations in habitat suitability are also identified in this work, which can help prioritize conservation areas, particularly the breeding areas that have remained stable over time, as this is a key requirement for little bustard lek breeding system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the patterns and processes that govern species distribution is critical for mitigating the widespread decline of biodiversity. This knowledge is essential for accurate and successful conservation planning (Guisan et al. 2013). In this context, Species Distribution Models (SDMs, similar to Ecological Niche Models; Elith and Leathwick 2009; Guisan et al. 2013) have gained considerable attention in recent decades, particularly given their utility for supporting biodiversity policy and decision processes (Araújo and Guisan 2006; Guisan et al. 2013). SDMs are based on the concept of the ecological niche (Ponti and Sannolo 2022), which describes the range of suitable biotic and abiotic conditions wherein a species is able to survive and reproduce (Colwell and Rangel 2009) as determined from species occurrence data. By identifying the most important environmental predictors of species occurrence, SDMs allow for the prediction of the species’ potential distribution across space and time (Elith and Leathwick 2009), thereby serving as key tools to support conservation and management initiatives.
Despite the many developments in the field, SDMs may be subject to some caveats related, for instance, to phenological variation in species’ ecological requirements, which may be blurred when using traditional approaches based on static correlative predictors, thus affecting the inferences that can be drawn from the data. Therefore, realistic and useful SDMs should reflect the seasonality related to species’ life-history events that drive their responses to spatial and temporal environmental change (Smeraldo et al. 2018). Although phenology is one of the most important factors in determining an organism’s ecological requirements, it has received little attention when modelling the potential distribution ranges of many species that exhibit spatial variation in habitat suitability across phenological phases (Smeraldo et al. 2018; Milanesi et al. 2020; Ponti and Sannolo 2022). The ecological requirements of migratory species, for instance, can change throughout the year, and their key environmental predictors at one phase may not be applicable at another (Chuine 2010). Additionally, to better understand the distribution patterns of such species over the long-term, the reconstruction of a species’ historic distribution range can be used to understand the influence of interannual environmental and climatic fluctuations (Ponti and Sannolo 2022). Yet, quantifying species distribution along phenological phases requires careful spatial and temporal matching of occurrence data with relevant environmental and climatic predictors (Elith and Leathwick 2009; Milanesi et al. 2020).
Matching high-resolution GPS animal tracking technology and satellite remote sensing products presents promising opportunities for the development of accurate SDMs over large spatial and temporal scales. Currently, GPS tracking technologies can provide a large number of an animal’s movement steps with a high spatial and temporal resolution for species of various sizes and ecologies (Nathan et al. 2008; Kays et al. 2015). Similarly, recent developments in satellite remote sensing provide the opportunity to affordably monitor environmental changes at fine spatial and temporal scales (Neumann et al. 2015). Remote sensing techniques currently provide access to topographic data, landscape biophysical and structural parameters, and climatic conditions (e.g., through general circulation models) with a resolution ranging from coarse to fine spatio-temporal scales (Kays et al. 2015). This makes remote-sensing products particularly good candidates for describing phenology-specific responses of species to environmental change (Cord et al. 2013).
For Steppe birds, which are among the most endangered terrestrial vertebrate species worldwide (BirdLife International 2022), we lack a thorough understanding the drivers of their distribution across key phenological phases. Such information is now urgently required to correctly infer their long-term declining trends and identify critically important conservation areas throughout the phenological cycle. In a European context, the Iberian Peninsula is known to support large populations of several threatened steppe and farmland bird species (Traba et al. 2013). However, due to habitat loss caused mostly by agriculture intensification, farmland birds have markedly declined in recent decades (Traba and Morales 2019; Silva et al. 2022). Among these, the little bustard (Tetrax tetrax) has shown particularly alarming declining trends, being globally classified as Near-Threatened (BirdLife International 2018) and as Vulnerable in Europe (BirdLife International 2021), including Spain (López-Jiménez et al. 2021). The Iberian Peninsula is a stronghold for the species, with the Extremadura region ranking among its most crucial conservation areas (Morales and Bretagnolle 2022). Conservation planning and effective management of little bustard populations throughout the annual cycle could greatly benefit from a precise, temporally and spatially explicit SDM.
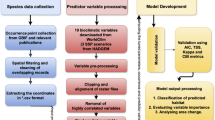
Here we used a 12-year GPS telemetry dataset from 91 tagged little bustards in southern Iberia and high resolution remotely sensed environmental metrics to generate seasonal SDMs (250 m resolution) regarding the key phenological phases of the species (e.g., Smeraldo et al. 2018). Our main aims were to: (i) identify the drivers of little bustard distribution and characterise the species’ response (in terms of probability of occurrence) to these, across its key phenological phases (winter, breeding, and post-breeding); and (ii) quantify the spatio-temporal variation (i.e., degree of stability) in habitat suitability across phenological phases and over the years (2005–2021). Overall, by considering phenology-related variations in the little bustard’s responses to environmental change across space and time, we expect our approach will provide novel insights on key ecological traits that are affecting long-term population trends.
Material and methods
Study area and study species
The Extremadura region, located in the southwest of Spain, is characterised by a meso-Mediterranean climate with warm, dry summers and cold, humid winters (Rivas-Martínez et al. 2002). Globally, the landscape of Extremadura is heterogeneous and fragmented, dominated by grazing and agriculture, with cereals and permanent crops grown on productive and irrigated plains. The region is subdivided into two provinces: Badajoz in the south and Cáceres in the north (Fig. 1). While Cáceres is characterised by pastures and semi-natural meadows with native oak forests and permanent pastures, Badajoz presents vast open areas that are predominantly made up of arable land.
Location of the study area in the Iberian Peninsula. The provinces of Cáceres and Badajoz are located north and south, respectively. The areas in grey show the potential geographical range for the little bustard, based on land cover classes taken from the European CORINE Land Cover. Special Protection Areas (SPAs) are shown by the polygons with black outlines (LC Llanos de Cáceres y Sierra de Fuentes; LT Llanos de Trujillo; LZ Llanos de Zorita y Embalse de Sierra Brava; LAB Llanos de Alcantara y Brozas; Ma Magasca; CS Campiña sur - Embalse de Arroyo Conejos; LS La Serena y Sierras Periféricas)
Several Special Protection Areas (SPAs) have been identified as priority sites for the preservation of the little bustard in Extremadura (Fig. 1). These areas represent the breeding grounds for roughly 65% of the population in the region (García De La Morena et al. 2018; Traba et al. 2022). Still, the little bustard has experienced a continued population decline over the last decades (López Ávila and Hidalgo de Trucios 1998; de Juana 2009), with a decrease in the Extremadura region of 33.2% in the winter population and 53.3% in the breeding population between the 2005 and 2016 national surveys (García De La Morena et al. 2018).
The annual cycle of the little bustard is subdivided into three distinct phenological phases: breeding, post-breeding, and winter (Silva et al. 2014, 2015). The species exhibits gregarious behaviour for the majority of the annual cycle, except for the breeding phase, when males establish territories (Jiguet et al. 2000). The Iberian population can be classified as partially migratory, with a small portion of individuals exhibiting strictly sedentary behaviour and the remainder displaying a range of migratory patterns. Food availability and environmental factors, such as ambient temperature, soil productivity, and vegetation height, are thought to have an impact on seasonal movements (Silva et al. 2007, 2015; García de La Morena et al. 2015). These patterns are typically synchronised to the phenological phases of the species, including regular movements that vary in timing and spatial range (García de La Morena et al. 2015).
Little bustard data
Little bustard presences and pseudo-absences were used to inform the SDMs. Presences were obtained from a 12-year GPS telemetry data set of 91 little bustards (all males, due to the challenges and difficulties of capturing females). Individuals were captured in Extremadura and Alentejo (Portugal) between 2009 and 2020 and fitted with highly precise GPS solar ARGOS Platform Transmitter Terminals (https://www.microwavetelemetry.com/) and solar GPS/GSM tags from Movetech Telemetry (https://movetech-telemetry.com/), E-Obs (https://e-obs.de/) and Ornitela OT (https://www.ornitela.com/) (e.g., Gudka et al. 2019). Data from Alentejo region were included in order to better inform SDMs (see Appendix S2). The data set was temporally filtered in order to obtain locations for the core periods of the three distinct phases of the little bustard yearly cycle: breeding (April 1–May 15), post-breeding (July 15–September 15), and winter (December 15–February 15). Then, we standardised the contribution of all the available individuals to avoid overrepresentation of some locations, as little bustard is highly site faithful during the different stages of the year (Alonso et al. 2019). For each bird, we selected a single biological phase per year and, within this phase, only a single location per day. Overall, 2214 locations of 41 individuals were used for the winter phase, 2225 locations of 82 individuals were used for the breeding phase, and 2877 locations of 59 individuals were used for the post-breeding phase, with 45.9% of these locations being in southern Portugal.
Pseudo-absences were generated in the same number of presences for each phenological phase (as recommended by Barbet-Massin et al. (2012) for Random Forests), following the distribution of land cover, at a minimum distance of 250 m to soften false absence error rates (Iturbide et al. 2015), and a maximum distance of 50 km for the breeding phase or 80 km for the post-breeding and winter phases (average distance of movements by phenological phase; Silva et al. 2014), to deal with possible inflated results (e.g., over-predictions) (see Appendix S2 for further details).
Predictor variables
As predictor variables of habitat suitability for little bustard during the different phenological phases, we included remote sensing products from optical (i.e., MODIS; or Moderate Resolution Imaging Spectroradiometer) and synthetic aperture radar (SAR) sensors (PALSAR1/2; or Phased Array Type L-Band Synthetic Aperture Radar) to infer key biophysical characteristics and habitat metrics from the spectral information, jointly with textural and structural variables to describe the “horizontal” and “vertical” complexity of the landscape. More conventional variables were also used in describing soil and topography, as well as human pressure (Table 1). Given the plethora of predictors considered, these were divided into biophysical, anthropogenic, and topographic predictors, each with a different spatial resolution, therefore subdivided into “static” (landscape “snapshots”) and “dynamic” (time-series) (Table 1). The calculation of variables was originally derived from multispectral remote sensing time series, that in turn allowed high-quality data agreement with telemetry observations (Milanesi et al. 2020), utilizing the Google Earth Engine (GEE) cloud platform (Gorelick et al. 2017).
In detail, satellite imageries were aggregated, corrected, and analysed, and the spatio-temporal concordance was addressed between calculated predictors and telemetry observations. These operations were carried out following the GEE_xtract framework presented in (Valerio et al. 2024), aiming to extract high-quality data, while providing an overview of the biophysical characteristics of agricultural and steppe habitats in the Mediterranean region. This involved landscape characteristics such as spectral data (red band—RED; near-infrared spectral band—NIR), vegetation conditions (Normalized Difference Vegetation Index—NDVI; Modified Soil Adjusted Vegetation Index 2—MSAVI2), biomass (Gross Primary Production—GPP), drought assessment (Palmer Drought Severity Index—PDSI) and land surface temperatures (LST) (Fernández et al. 2010; Cerasoli et al. 2018; von Keyserlingk et al. 2021; Valerio et al. 2023) (Table S1 from Appendix S1). To infer vegetation’s horizontal structure, Gray-level co-occurrence matrix (GLCM) indices (Haralick et al. 1973) were calculated through the NDVI, providing summary statistics of texture patterns, such as mean (GLCM_M) and variance (GLCM_V), as well as contrast metrics, including homogeneity (GLCM_H) and contrast (GLCM_C) (Fernández et al. 2010; Wood et al. 2012). Additionally, yearly mosaics of L-band SAR data with combined polarizations (horizontal transmitting/horizontal receiving—HH polarization; horizontal transmitting/vertical receiving—HV polarization) from ALOS PALSAR satellites were used to infer the vertical structure of vegetation (Lucas et al. 2010). To incorporate information regarding the spatial density distribution of artificially sealed areas, an indicator of human imperviousness (IMD) was obtained from the Copernicus programme (Copernicus Land Monitoring Service, 2021), in addition to the Corine land cover classes (CLC) which were used as categorical predictors. Moreover, the modelling process incorporated a collection of static predictors as well, which relate to relatively stable landscape characteristics. These predictors encompassed: (i) soil properties (pH; sand content—SC; organic carbon density—OCD; bulk density—BD; Poggio et al. 2021); (ii) bioclimatic conditions (annual mean temperature—BIO1; mean diurnal range—BIO2; annual precipitation—BIO12; precipitation of driest month—BIO14; Fick and Hijmans 2017); (iii) as well as topographic attributes (altitude, slope, terrain ruggedness index—TRI, and wetness index—TWI; Conrad et al. 2015; Crippen et al. 2016); and (iv) anthropogenic information (distance from major roads—Dist_MR; distance from power lines—Dist_PL; GeoFabrik 2021). All topographic variables were calculated using the equation provided in Table S1 (Appendix S1) through the software SAGA GIS (v.2.1.4; Conrad et al. 2015). Further descriptions of predictors’ environmental meaning and biological rationale can be found in Tables 1 and S1, respectively.
Habitat suitability modelling
SDMs were employed to determine the variation in habitat suitability for the little bustard both spatially across phenological phases (seasonal SDMs) and temporally over the years. We used presences and pseudo-absences as response variables and both dynamic and static predictors as explanatory variables. Following the parametrisation in Valerio et al. (2020), models were run as probabilistic classifications by selecting Random Forests (Breiman 2001), for which the variant algorithm "Boruta" (R package v.6.0.0; Kursa and Rudnicki 2010) was used for a prior screening procedure to identify and filter out irrelevant predictors. The selection process relied on analyses that determined “confirmed” and “rejected” predictors by comparing the importance of predictors with that of their randomised copies, in which values were shuffled (Kursa and Rudnicki 2010).
Each multivariate SDM with retained predictors was developed using tenfold cross-validations, and evaluated using a set of five accuracy metrics derived from the confusion matrix. These metrics included sensitivity, which measures the proportion of correctly classified occurrences, and specificity, which assesses the proportion of correctly classified absences (Fielding and Bell 1997). Both metrics varies from 0 to 1, with values > 0.9 being indicative of good to excellent discriminant accuracy (Plante and Vance 1994). Additionally, we included the area under the receiver operating characteristic (ROC) curve, often referred to as AUC (Swets 1979). Here, values near 1 indicate a high level of predictability by the model (e.g., Araújo et al. 2005). To further enhance the evaluation process, the Boyce Index (Boyce et al. 2002) was integrated, with calculations carried out using the "modEvA" R package (v.3.9; Barbosa et al. 2013). This index varies from − 1 to 1, with values close to 1 indicating that the model's predictions are consistent with the presences’ distribution (Jiménez and Soberón 2020). To complement the evaluation, we also included the Matthews' correlation coefficient (MCC; Matthews 1975; Baldi et al. 2000). The inclusion of MCC is particularly valuable due to its robustness, as it considers all categories of the confusion matrix, encompassing true positives, true negatives, false positives, and false negatives. This metric also varies from − 1 to 1, with values of 1 indicating perfect classification (Chicco and Jurman 2020).
Finally, to distinguish between suitable and unsuitable areas, the continuous probability maps were converted to binary using a cutoff. This cutoff was calculated independently for each phenological phase as the average of four different threshold selection methods. The methods we used include: (i) minimizing the absolute difference between sensitivity and specificity (SeSpeql); (ii) maximizing the sum of sensitivity and specificity (SeSpmax); (iii) maintaining the original prevalence (PredPrev = Obs); and (iv) taking the mean of the probabilities of occurrence of occupied locations for presence/absence data as the threshold (AvgProb) (see Liu et al. 2005, 2013; Nenzén and Araújo 2011, for detailed explanation). The models were subsequently projected for the Extremadura region between 2005 and 2021, covering the period between the first Spanish national census and the present. For detailed information on the modelling process, see Appendix S2.
Spatio-temporal variation in habitat suitability
Spatial variation in habitat suitability across phenological phases
The median habitat suitability (HS), which reflects the species' expected probability of occurrence, was calculated for the last 3 years (2019–2021) in order to evaluate the current habitat suitability for the little bustard at each phenological phase. To visualise the spatial variation in habitat suitability between consecutive phenological phases, we calculated the difference between the HS values of a given phase minus those of the next phase. This allowed us to determine the variations in suitability at each location. Then, the percentage of suitable habitat (HS > cutoff; specific for each phenological phase) overlap between phases was calculated, as well as the pairwise niche overlap using Schoener’s D (Schoener 1968) with the function “raster.overlap” from the R package “ENMTools” (Warren et al. 2010). This metric, which compares the corresponding values for each cell in two grids to determine how similar potential distributions are, ranges between 0 (no similarity) and 1 (identical potential distribution) (Broennimann et al. 2012).
Temporal variation in habitat suitability
To assess the stability in habitat suitability over time, we estimated, for each phenological phase, the coefficient of variation of the HS between 2005 and 2021, calculated for each pixel and expressed as a percentage, using the “CV” function from the R package “raster” (Hijmans 2023). This enabled the most stable zones to be distinguished from those with greater variation in suitability over the period under consideration. Then, to visualise the temporal progression of sites with suitable habitat (HS > cutoff, specific for each phenological phase) between 2005 and 2021, we compared the baseline situation (HS median between 2005 and 2007) with the current situation (HS median between 2019 and 2021). The areas where suitable habitat decreased or increased, as well as the areas that remained stable above the suitability threshold, were then identified and measured for each phenological phase. Finally, to estimate the fluctuations in the availability of suitable habitat (HS > cutoff) in each phenological phase over the period 2005–2021, we calculated the area covered by all pixels classified as suitable for the species in each phase of each year.
Results
Seasonal little bustard SDMs
Explanatory predictors grouped as “dynamic” were among the most relevant variables in our seasonal SDM approach, in particular those referred to as landscape horizontal structure (textural) predictors (GLCM_C and GLCM_V, respectively NDVI-based texture contrast and variance metrics) jointly with those describing vertical structure (HV polarization) (Fig. 2). Conversely, the most influential static predictors were those describing soil properties (sand content—SC), bioclimate (annual precipitation—Bio12; annual mean temperature—Bio1; and precipitation of driest month—Bio14), and topography (terrain ruggedness index—TRI; and terrain water index—TWI). Both the importance scores of the predictors in explaining the distribution of little bustards (Fig. 2) and their ecological response curves (see Fig. S3 in Appendix S1) vary between phenological phases. The NDVI-based texture contrast (GLCM_C) was the most important predictor during the winter phase and displayed a positive relationship with little bustard locations, where contrast in vegetation characteristics contributed to increase the probability of occurrence of the species, similar to the NDVI-based texture variance (GLCM_V). This effect was less significant during the post-breeding phase (Fig. 2), and relationships become negative during the breeding phase (see Fig. S3 in Appendix S1). The HV polarization (HV) was also an important predictor during the winter and breeding phases, where the lower the values of HV (relating to herbaceous vegetation), the higher the probability of little bustard occurrence. In relation to the soil sand content (SC) predictor, there was evidence for a relatively higher probability of species occurrence within areas of low sand content values, which is likely related to more productive soils. Conversely, when SC exceeds 35% (weight%), the little bustard appears to avoid these soils. The topographic predictors, namely terrain ruggedness index (TRI) and terrain water index (TWI), held higher importance scores during both the post-breeding and winter phases, maintaining a stable negative (low topographic heterogeneity) and positive (higher water potential accumulation) relationship with little bustard occurrence, respectively. The bioclimatic predictors were more significant during the post-breeding phase (Bio1 and Bio12) and breeding (Bio1 and Bio14), with Bio12 (Annual Precipitation) and Bio14 (Precipitation of Driest Month) exerting a positive effect, while Bio1 (Annual Mean Temperature) exerting a positive and a negative effect during the post-breeding and breeding phases, respectively (Figs. 2 and S3).
Importance scores, in explaining the little bustard distribution from random forests analysis for each phenological phase. The green dot indicates that the predictor was significant in the previous screening procedure (Boruta). The symbols “+” and “−” are attributed to the top five most relevant predictors and refer to whether the response curve was positive or negative. Response curves from partial dependence plots may be found for all predictors in Appendix S1 (Fig. S3)
All SDMs demonstrated high predictive power (Fig. 3). In detail, the set of observations representing the most predictive phenological phase was breeding for all accuracy metrics, except for the Boyce index, where the most predictive phase was post-breeding. Excellent performances were observed, given the high AUC scores (> 0.95), with no apparent differences between phases. High abilities were also found in predicting true presences (sensitivity > 0.9) and false absences (specificity > 0.9), though slightly better performances were detected in predicting true presences during breeding than in other phases. The MCC metric results indicated that most models performed with high accuracy scores (> 0.85), as well as the Boyce index (> 0.9) (Fig. 3).
Differences in SDMs’ performances across datasets representing distinct phenological phases. Model performance results are shown according to selected accuracy metrics: area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and Matthews’ correlation coefficient (MCC). Boxplots and grey dot points show the performance of cross-validation repetitions of random forests analyses, while black dots represent the mean performance
Spatio-temporal variation in habitat suitability
Spatial variation in habitat suitability across phenological phases
Habitat suitability maps of the current situation (2019–2021; Fig. 4a) suggest a clear change in the spatial distribution of suitable habitat among the three phenological phases. The distribution of the most suitable locations generally coincides with the interior of SPAs and their interface with the surrounding areas, during both the breeding and winter phases. Nonetheless, during the breeding phase, suitable areas are mostly concentrated in the eastern part of the province of Badajoz’s, while during the winter phase, additional favourable habitats also can be found in the province of Cáceres’ southern and central parts. The northwest of Cáceres and the southwest of Badajoz stand out as areas of lower suitability during both the breeding and winter phases. On the other hand, the post-breeding phase shows a more pronounced variation in the distribution of suitable areas. The areas of higher suitability during this phase are primarily located outside SPAs and are dispersed more widely across the study region (Fig. 4a). The main suitable area during the post-breeding phase is located along the banks of the Guadiana River in the Extremadura region’s central zone. The central and northwest zones of the province of Cáceres as well as the southeast region of the province of Badajoz also stand out for their high levels of suitability. In contrast, one of the least suitable areas during this phase is the Badajoz province’s east central region.
a Current habitat suitability situation; b spatial variation in habitat suitability between consecutive phenological phases; c stability in habitat suitability over time (coefficient of variation of the HS between 2005 and 2021; truncated to 30 for visualisation purposes); d temporal progression of sites with suitable habitat (long-term changes from the baseline to the current period). The black-outlined polygons delineate the boundaries of the two provinces, while the white-outlined polygons delineate the Special Protection Areas (SPAs)
Despite the minor seasonal variation of suitable habitat between the winter and breeding phases, differences were more noticeable inside SPAs, which have slightly decreased suitability indices, and in the north and south-central zones of the region, which have slight gains in habitat suitability (Fig. 4b). Transitions involving the post-breeding phase are associated with stronger variation in suitability. As for the breeding phase transitions to the post-breeding phase, there is an increase in suitability in the areas surrounding the Guadiana River, in the central and northern areas of the province of Cáceres, as well as in the southeast area of the province of Badajoz. On the other hand, the central west and east zones of the provinces of Cáceres and Badajoz, respectively, exhibit a downward variation in suitability indices. As expected, given the similarity between the breeding and winter phases, the spatial variation of suitability in the breeding—post-breeding transition is opposite to that in the post-breeding—winter transition (Fig. 4b).
The averaged suitability cutoff, used to identify suitable areas (HS value > cutoff), differs for each phenological phase (winter: 0.524; breeding: 0.500; post-breeding: 0.492; see Table S3 from Appendix S1). In the current situation (2019–2021), this translates to 1616.93 km2 of available suitable habitat during the winter, 2100.51 km2 during breeding, and 4500.98 km2 during post-breeding. The seasonal overlap of suitable habitat is ultimately impacted by these differences in the availability of suitable areas. Despite the winter and breeding phases sharing only 681.99 km2 of suitable areas, they show the highest similarity in the distribution of their HS values (D = 0.95), indicating a high spatial concordance in the suitability of habitats during the winter and breeding phases. Conversely, although the shared suitable area between breeding and post-breeding phases is substantially larger (1177.12 km2), the distribution of their HS values is less similar (D = 0.85), indicating spatial discrepancy in the suitability of habitats between the breeding and post-breeding phases (Table 2). The post-breeding and winter phases, with an overlap of 885.3 km2 of suitable areas, have the least similar distribution of HS values (D = 0.82). With regard to the location of suitable habitat in relation to SPAs, the winter phase has the highest percentage of suitable habitat within these areas (52.38%), followed by the breeding phase (38.36%), with the post-breeding phase presenting the lowest percentage (20.36%) (Table 3).
Temporal variation in habitat suitability
Over the study period (2005–2021), zones near water reservoirs and water lines, which are typically unsuitable for little bustards, were those showing the greatest instability (i.e., greatest coefficient of variation) in the indices of habitat suitability (Fig. 4c). Low to moderate instability was observed during the winter and breeding phases across the study area, with these zones mainly being connected to SPAs and their surroundings, which are typically suitable for the species. In both phases, the areas closest to the Guadiana River and in the northwest of the province of Cáceres are the most stable over time, despite not being suitable for the species during these phases. In contrast, the post-breeding phase displayed lesser stability overall, with the zones of greatest instability typically being those that were unsuitable for the species. However, similarly to other phases, low to moderate instability was observed in areas suitable for little bustards during the post-breeding phase (Fig. 4c).
Comparing the baseline situation (2005–2007) with the current winter phase situation (2019–2021) in terms of the availability of suitable habitat (HS > cutoff), a net gain of 828.91 km2 (corresponding to 105.19% of the suitable area in the baseline situation) was recorded (Table 3). However, about 36.65% of the area that was suitable in the baseline situation is no longer suitable. These losses in suitability occurred mainly in the vicinity of the Guadiana River, whereas suitability gains were mainly registered in the interior and interface areas of the SPAs, primarily in the province of Badajoz (Fig. 4d). It is also worth noting that suitable habitat is more stable inside SPAs (77.33%) than it is outside (63.35%) (Table 3). In the breeding phase, there was a 23.31% decrease in the available suitable area (a net loss of 638.42 km2), with only 56.44% of the suitable area in the baseline situation remaining suitable (Table 3). Still, inside SPAs, the decrease in the available suitable area was smaller (12.02%) and that stability was higher (64.06%). The areas that remained stable were found mainly in the south and east of the province of Badajoz. The losses occurred in the vicinity of the Guadiana River and the central south zone of the province of Cáceres, as well as in the interface areas of the SPAs of Badajoz (Fig. 4d). In the post-breeding phase, there was a slight overall increase in the availability of suitable habitat between the baseline and current situations (9.65%; a net gain of 396.2 km2), with 59.49% of the suitable habitat at the baseline remaining stable (Table 3). However, within SPAs the situation is quite different, registering a 23.50% decrease in the availability of suitable areas (a net loss of 281.5 km2) and a stability of only 52.03% of the suitable areas. While gains were primarily recorded in the central zone of the study area and the northern zone of the province of Cáceres, the majority of suitability losses occurred in the south and east of the province of Badajoz and the southwest zone of the province of Cáceres (Fig. 4d). The Guadiana River area and the southeast of Badajoz province are the main locations where the habitat remained suitable.
Over time, the winter phase typically displayed the most spatially constrained area of suitable habitat, while the post-breeding phase consistently displayed the broader area of suitable habitat most of the time, according to the analysis of the predicted area of suitable habitat over the period 2005–2021 (Figs. 5; S5b, S7). Large fluctuations in the availability of suitable habitats were observed over the period considered, mostly during the breeding and post-breeding phases. These fluctuations were not always synchronous between phenological phases, as was the case, for instance, between 2010 and 2013, when there was a tendency for habitat availability to decrease during the breeding phase and increase during the winter and post-breeding phases (Fig. 5). Overall, a positive trend in the availability of suitable habitat is observed during the winter phase, whereas an apparent negative trend is observed during the breeding phase. The post-breeding phase does not show any apparent trend, despite the large fluctuations over time.
Discussion
Based on a detailed analysis of the spatial and temporal variation in habitat suitability of the little bustard, our study demonstrated clear seasonal variations in the spatial distribution of suitable habitat for the species along key phenological phases. Additionally, results also showed marked fluctuations in suitability over the past 17 years. When comparing the current situation to the baseline, we found an increase in suitability during the winter phase, a slight increase during the post-breeding phase, and a reduction in suitability during the breeding phase. Furthermore, our study also allowed the identification of locations where the habitat remains suitable over time, contributing to the definition of areas of high conservation value in future conservation planning (Silva et al. 2017).
Our study showed that the use of data with high temporal and spatial resolution from GPS telemetry and remote sensing, together with machine learning modelling procedures, allowed for a robust assessment of variation in species-specific habitat suitability along distinct phenological phases, as well as the prediction of the present and past potential distribution of migratory species. This methodology thus contributes to a thorough understanding of the dynamics in species potential distribution ranges over multiple phenological phases, as well as the identification of the most important habitat variables that predict species occurrence and on which conservation efforts should focus.
Potential distribution of the little bustard with seasonal SDMs
The accurate SDMs produced for each phenological phase showed that high-quality data used as input to Random Forest algorithms may present opportunities for providing information on the geographic distribution of species. Both static and dynamic predictors were found among the most explanatory variables in describing species occurrence, highlighting the relevance of including both types of predictors together with the modelling of each phenological phase separately (Frans et al. 2018). It should be noted that all of the CORINE Land Cover products fell below the mean importance threshold (Fig. 2), highlighting the benefits of less conventional products with both SAR and optical information to highlight scarcely represented habitats (Valerio et al. 2020).
The winter model indicated that there is a greater probability for little bustards to occur within agricultural mosaics where herbaceous vegetation predominates, the soils are more productive, the topography is less rugged, and there is a tendency for very low or moderately high levels of water to accumulate. The representation of landscape mosaic in our analyses is supported by our textural predictors (i.e., NDVI-based texture contrast—GLCM_C) describing horizontal landscape complexity, since a positive response was found for contrasting NDVI values, given also the scale of the sensor grain (250 m) and the window for the considered adjacent neighbour pixel. Concomitantly, the negative response for high backscattering values (i.e., HV polarization) supports the presence of a moderate vertical complexity of vegetation, since high backscatter values are associated with taller and more structured vegetation such as shrubs and trees (e.g., Valerio et al. 2023). These preferences are consistent with other studies showing that, during this phenological phase, little bustards select recent fallows and grassy vegetation, as well as hilltops, possibly as a predator avoidance strategy (Silva et al. 2004).
During breeding, little bustards showed a preference for areas dominated by herbaceous vegetation within more homogeneous landscapes, productive soils, intermediate levels of rainfall in the driest month, and moderate annual mean temperatures. These preferences are consistent with previous works showing a preference for vast expanses of grassland pastures or fallow lands with low land cover diversity, and a dominant grassland ecosystem (Morales et al. 2008; Silva et al. 2010; Moreira et al. 2012). Such choices are probably related to the species' lek mating system, in which breeding males seek conspicuousness for the sexual displays that take place in loose aggregations, whereas females seek a balance between visibility for anti-predator surveillance and cover provided by dense vegetation (Jiguet et al. 2000).
The most suitable areas for post-breeding, according to our model, coincide with depressions, where it is more likely to accumulate water and green vegetation that they feed on. Their occurrence also coincides with regions with higher average temperatures and higher annual precipitation, as well as some degree of heterogeneity in the landscape. Again, these preferences are in line with previous works that show a preference towards areas near water sources, on lower slopes, with more humidity, and with more green plants (Silva et al. 2007). Food availability is suggested to play a significant role in habitat selection and species distribution during post-breeding (Silva et al. 2007), with adults and chicks feeding mostly on green plants, in the period defined for post-breeding in our models (July 15–September 15) (Jiguet et al. 2002).
Spatial variation in habitat suitability across phenological phases
According to our models, the extent and distribution of suitable habitat vary between phenological phases. During the winter phase, the area with suitable habitat is narrower and spatially more clustered, whereas it expands and spreads more widely during the breeding phase until it reaches its maximum extent in the post-breeding phase (Figs. 4a, S5b). These seasonal variations appear to reflect the behaviour of the little bustard, which exhibits territorial behaviour during breeding (Silva et al. 2017) and gregarious behaviour in the remaining phases (Silva et al. 2004, 2007). Outside the breeding season, little bustards congregate in flocks of varied sizes, but it is during the winter that they are more concentrated, creating the largest flocks.
The species’ behavioural strategy appears to be influenced by food availability. During the winter, when there is plenty of food, the species shows greater habitat selectivity, which is possibly related to an anti-predatory strategy, increasing the level of aggregation in the most suitable habitats as the season progresses, providing food and protection (Silva et al. 2004; Morales et al. 2022). In the breeding phase, breeding males form dispersed leks at sites that were used in previous years (Silva et al. 2017), disperse over larger areas compared to the winter phase. Major shifts in the little bustard’s distribution occur during post-breeding. This is probably because at the end of the breeding season, in late spring, vegetation dries out, restricting the little bustard’s food resources and forcing individuals to migrate towards areas with more productive soils, which frequently coincide with irrigated fields with greater availability of green plants (Silva et al. 2007, 2022; García de La Morena et al. 2015). At this phase, flocks are usually small, ranging from a few birds to several tens, while occupying a wider range of habitats (Silva et al. 2007). There is a greater similarity in potential distribution of suitable habitat between the winter and breeding phases when compared to the post-breeding phase, suggesting that the scarcity of food during the summer leads to a change in occurrence patterns (Fig. 4b, Table 2).
The distribution of suitable habitats during the winter and breeding phases greatly overlaps with the SPAs. Conversely, the most suitable areas for post-breeding are found outside SPAs, principally in the irrigation fields next to the Guadiana River, with only about 20% of these areas occurring within protected areas. On the other end of the spectrum is the winter phase, which has 52% of its total suitable area inside SPAs. In turn, the breeding phase presents about 38% of its suitable area inside SPAs, highlighting the importance of these areas for the conservation of the species, as demonstrated by the surveys done in Extremadura, which revealed that 65% of the breeding males are present inside SPAs (García De La Morena et al. 2018). When comparing the provinces of Badajoz and Cáceres in terms of the spatial distribution of suitable habitat, Badajoz has higher overall suitability, demonstrating greater availability of suitable habitat at all phenological phases. This results from the differences in landscape characteristics between the two provinces, with Badajoz having a higher availability of open habitats compared to Cáceres.
The fact that our study relied on GPS data provided solely by males may be considered a limitation. However, because females tend to occur in areas close to males (except for the chick-rearing period that was not included within the breeding phase considered in our analysis) (Silva et al. 2014; Morales et al. 2022), we did not expect that male and female preferences would differ at the level at which we analysed the data (Devoucoux et al. 2018).
Temporal variation in habitat suitability
Over the last two decades, the little bustard population from Extremadura has experienced a sharp decline, dropping as much as 53% between 2005 and 2016 (García De La Morena et al. 2018), a trend that is ongoing (SEO BirdLife, pers. comm.). This trend coincides with a gradual loss of suitability over time (Figs. 4d, 5, and Table 3), particularly in the breeding phase, when the amount of suitable habitat for the species dropped by 23% over the period considered (2005–2021). Noticeably, only 56% of the breeding habitat remained stable during this period, which is a known requirement for viable breeding areas due to the species’ high breeding site fidelity (Silva et al. 2017), highlighting the possible negative effects of the instability observed over time. However, despite the high level of instability, the availability of suitable habitat increased during the winter and, to a lesser extent, in the post-breeding phases too. These findings suggest that while the available winter and post-breeding habitats do not appear to pose a limitation for the species' conservation in Extremadura, the reduced availability of breeding sites may be acting as a bottleneck during a critical period in the population dynamics of this species. Even though there is considerable uncertainty with the demographic parameters of the breeding population of Extremadura, there is evidence suggesting that habitat loss and degradation, along with climate change, particularly during the breeding season, have adversely affected both the productivity and survival of females (Silva et al. 2022). Furthermore, there is a notable issue of high adult mortality associated with power lines (Marcelino et al. 2018).
While the long-term temporal variation in habitat suitability can be related mainly to land-use conversion (Silva et al. 2022), the large fluctuations in the availability of suitable habitat recorded between years (Fig. 5) and the variations in average suitability (see Fig. S5 in Appendix S1) may be related to the inter-annual variation in climatic and biotic conditions (i.e., vegetation condition) (García de La Morena et al. 2015; Estrada et al. 2016). This spatial variation in suitable areas may have serious impacts on the species since little bustards have marked philopatric habits, returning to the same places in consecutive years (Silva et al. 2017; Alonso et al. 2019). In this sense, it is also noteworthy that new potential habitats may not be immediately occupied, as this implies an additional energy expenditure when birds actively search for them (Holt 2003).
The analysis of the temporal variation of habitat suitability highlighted the importance of SPAs for the little bustard, particularly during the breeding and winter phases. In addition to the higher average HS values over time within the SPAs, the stability of suitable habitat was also higher within these areas during these two phenological phases when compared to the non-SPA areas. Furthermore, in percentage terms, less habitat was lost inside the SPAs during these two phases, which may indicate that they are buffering against habitat loss and degradation up to a certain extent. However, although in post-breeding there was an overall gain in suitable habitat, within the SPAs the pattern was opposite, with important losses being recorded. These results may be, at least in part, explained by the fact that SPAs were designed with a focus on preserving breeding areas and by the land-use conversion restrictions existing in these protected areas.
It is worth mentioning, however, that results on how habitat suitability changes over space and time are intrinsically linked to the cutoff value used to generate the binary maps. This value changes with each phenological phase and affects the area that is considered to be suitable (> cutoff) or unsuitable (< cutoff), influencing the areas of overlap between phenological phases and the trends over time.
Conservation implications
The research presented here demonstrates fluctuations in the habitat suitability of the little bustard over time considering all phenological phases, and an apparent decline in the breeding phase. These results support the hypothesis that, at the population level, habitat instability and degradation are contributing towards the species decline, given the species' high fidelity to the same locations between years. Our modelling procedure identified most important dynamic predictors defining the species’ phenological niches. These predictors were found to be mainly related to the structural characteristics of the habitat, rainfall rates, and average air temperatures, making the species vulnerable not only to habitat shifts but also to climate change.
In terms of conservation, priority should therefore be given to the promotion of high-quality habitat through the encouragement of traditional extensive agricultural practices, which primarily provide important habitat during the breeding and winter phases (Silva et al. 2004, 2010). Habitat stability over time is critically important to ensure high breeding densities and consequently breeding success by favouring its lekking breeding system (Silva et al. 2014, 2017). The results also highlight the importance of SPAs in the conservation of little bustards, especially during winter and breeding, given the apparent buffer effect they exert against habitat suitability loss and the greater proportion of habitat suitability stability found within these phenological phases. Taking into account the amount of suitable habitat found at the interface between the SPAs and the areas outside them, expanding these special protection areas could be beneficial for the species.
Our modelling procedure and the predictions regarding bird occurrence associated with the calculated cut-off value indicate that the changes in suitability vary across both time and space. These changes, however, significantly align with already identified breeding areas, including those deemed critically important as Special Protection Areas (SPAs), alongside post-breeding and wintering locations. The models developed here can therefore serve as a crucial decision-support tool for conservation efforts, by providing accurate, spatially explicit probability estimates of little bustards’ current and historical occurrence as well as details on the key environmental factors affecting the species at various phenological phases. Highly suitable areas that show stability over time should be considered of high conservation priority, particularly during the breeding phase. Overall, our approach offers relevant complementary information to existing research on the ecology and conservation of the little bustard. This information is particularly important for contextualizing the factors contributing to the species' decline over time in a spatially explicit manner, thereby facilitating integrated decision-making.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alonso H, Correia RA, Marques AT, Palmeirim JM, Moreira F, Silva JP (2019) Male post-breeding movements and stopover habitat selection of an endangered short-distance migrant, the Little Bustard Tetrax tetrax. Ibis. https://doi.org/10.1111/ibi.12706
Araújo MB, Guisan A (2006) Five (or so) challenges for species distribution modelling. J Biogeogr 33:1677–1688
Araújo MB, Pearson RG, Thuiller W, Erhard M (2005) Validation of species-climate impact models under climate change. Glob Chang Biol 11:1504–1513
Baldi P, Brunak S, Chauvin Y, Andersen CAF, Nielsen H (2000) Assessing the accuracy of prediction algorithms for classification: an overview. Bioinformatics 16:412–424
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338
Barbosa AM, Real R, Muñoz AR, Brown JA (2013) New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers Distrib 19:1333–1338
BirdLife International (2018) Tetrax tetrax. In: The IUCN Red List of Threatened Species 2018: e.T22691896A129913710. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22691896A129913710.en. Accessed 8 Dec 2022
BirdLife International (2021) European red list of birds. Publications Office of the European Union, Luxembourg
BirdLife International (2022) State of the World’s Birds 2022: Insights and solutions for the biodiversity crisis. BirdLife International, Cambridge
Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA (2002) Evaluating resource selection functions. Ecol Modell 157:281–300
Breiman L (2001) Random forests. Mach Learn 45:5–32
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin MJ, Randin C, Zimmermann NE, Graham CH, Guisan A (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21:481–497
Cerasoli S, Campagnolo M, Faria J, Nogueira C, da Conceição Caldeira M (2018) On estimating the gross primary productivity of Mediterranean grasslands under different fertilization regimes using vegetation indices and hyperspectral reflectance. Biogeosciences 15:5455–5471
Chicco D, Jurman G (2020) The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21:1–13
Chuine I (2010) Why does phenology drive species distribution? Philos Trans R Soc B 365:3149–3160
Colwell RK, Rangel TF (2009) Hutchinson’s duality: the once and future niche. Proc Natl Acad Sci USA 106:19651–19658
Conrad O, Bechtel B, Bock M, Dietrich H, Fischer E, Gerlitz L, Wehberg J, Wichmann V, Böhner J (2015) System for automated geoscientific analyses (SAGA) v. 2.1.4. Geosci Model Dev 8:1991–2007
Cord AF, Meentemeyer RK, Leitão PJ, Václavík T (2013) Modelling species distributions with remote sensing data: bridging disciplinary perspectives. J Biogeogr 40:2226–2227
Crippen R, Buckley S, Agram P, Belz E, Gurrola E, Hensley S, Kobrick M, Lavalle M, Martin J, Neumann M, Nguyen Q, Rosen P, Shimada J, Simard M, Tung W (2016) Nasadem global elevation model: methods and progress. Int Arch Photogr Remote Sens Spatial Inf Sci 41:125–128
de Juana E (2009) The dramatic decline of the little bustard Tetrax tetrax in extremadura (Spain). Ardeola 56:119–125
Devoucoux P, Besnard A, Bretagnolle V (2018) Sex-dependent habitat selection in a high-density Little Bustard Tetrax tetrax population in southern France, and the implications for conservation. Ibis 161:310–324
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Estrada A, Delgado MP, Arroyo B, Traba J, Morales MB (2016) Forecasting large-scale habitat suitability of European bustards under climate change: the role of environmental and geographic variables. PLoS ONE 11:1–17
Fernández N, Paruelo JM, Delibes M (2010) Ecosystem functioning of protected and altered Mediterranean environments: a remote sensing classification in Doñana, Spain. Remote Sens Environ 114:211–220
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Frans VF, Augé AA, Edelhoff H, Erasmi S, Balkenhol N, Engler JO (2018) Quantifying apart what belongs together: a multi-state species distribution modelling framework for species using distinct habitats. Methods Ecol Evol 9:98–108
García de La Morena EL, Morales MB, Bota G, Mañosa S, Morales M (2015) Migration patterns of Iberian little bustards Tetrax tetrax. Ardeola 62:95–112
García De La Morena EL, Bota G, Mañosa S, Morales M (2018) El Sisón Común en España. II Censo Nacional. SEO/Birdlife. Madrid, Madrid
GeohjFabrik. GeoFabrik: Download Server for OpenStreetMap data. 2021. Web Based Download Application: Available online: http://download.geofabrik.de/ accessed 20 Oct 2021
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google earth engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27
Gudka M, Santos CD, Dolman PM, Abad-Gómez JM, Silva JP (2019) Feeling the heat: elevated temperature affects male display activity of a lekking grassland bird. PLoS ONE 14:e0221999
Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Tulloch AIT, Regan TJ, Brotons L, Mcdonald-Madden E, Mantyka-Pringle C, Martin TG, Rhodes JR, Maggini R, Setterfield SA, Elith J, Schwartz MW, Wintle BA, Broennimann O, Austin M, Ferrier S, Kearney MR, Possingham HP, Buckley YM (2013) Predicting species distributions for conservation decisions. Ecol Lett 16:1424–1435
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern 3:610–621
Hijmans R (2023) raster: geographic data analysis and modeling. R package version 3.6–26. https://rspatial.org/raster
Holt RD (2003) On the evolutionary ecology of species’ ranges. Evol Ecol Res 5:159–178
Iturbide M, Bedia J, Herrera S, del Hierro O, Pinto M, Gutiérrez JM (2015) A framework for species distribution modelling with improved pseudo-absence generation. Ecol Modell 312:166–174
Jiguet F, Arroyo B, Bretagnolle V (2000) Lek mating systems: a case study in the Little Bustard Tetrax tetrax. Behav Process 51:63–82
Jiguet F, Jaulin S, Arroyo B (2002) Resource defence on exploded leks: do male little bustards, T. tetrax, control resources for females? Anim Behav 63:899–905
Jiménez L, Soberón J (2020) Leaving the area under the receiving operating characteristic curve behind: an evaluation method for species distribution modelling applications based on presence-only data. Methods Ecol Evol 11:1571–1586
Kays R, Crofoot MC, Jetz W (2015) Terrestrial animal tracking as an eye on life and planet. Science (1979) 348:2478
Kursa MB, Rudnicki WR (2010) Feature selection with the boruta package. J Stat Softw 36:1–13
Liu C, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28:385–393
Liu C, White M, Newell G (2013) Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr 40:778–789
López Ávila P, Hidalgo de Trucios S (1998) Revisión del status del Sisón: Evolución en Extremadura. In: Junta de Extremadura (ed) Conservación de la naturaleza y los espacios protegidos de Extremadura. Mérida, pp 115–121
López-Jiménez N, García de la Morena E, Bota G, Mañosa S, Morales MB, Traba J (2021) Sisón Comun, Tetrax tetrax. In: López-Jiménez N (ed) Libro Rojo de las Aves de España. SEO/BirdLife, Madrid, pp 521–527
Lucas R, Bunting P, Clewley D, Armston J, Fairfax R, Fensham R, Accad A, Kelley J, Laidlaw M, Eyre T, Bowen M, Carreiras J, Bray S, Metcalfe D, Dwyer J, Shimada M (2010) An Evaluation of the ALOS PALSAR L-Band Backscatter—above ground biomass relationship queensland, australia: Impacts of surface moisture condition and vegetation structure. IEEE J Sel Top Appl Earth Obs Remote Sens 3:576–593
Marcelino J, Moreira F, Mañosa S, Cuscó F, Morales MB, García De La Morena EL, Bota G, Palmeirim JM, Silva JP (2018) Tracking data of the Little bustard Tetrax tetrax in Iberia shows high anthropogenic mortality. Bird Conserv Int 28:509–520
Matthews BW (1975) Comparison of the predicted and observed secondary structure of T4 phage lysozyme. BBA Protein Struct 405:442–451
Milanesi P, Della Rocca F, Robinson RA (2020) Integrating dynamic environmental predictors and species occurrences: toward true dynamic species distribution models. Ecol Evol 10:1087–1092
Morales MB, Bretagnolle V (2022) The little bustard around the world: distribution, global conservation status, threats and population trends. In: Bretagnolle V, Traba J, Morales MB (eds) Little bustard: ecology and conservation, wildlife R. Springer, Cham, pp 57–80
Morales MB, Traba J, Carriles E, Delgado MP, de la Morena ELG (2008) Sexual differences in microhabitat selection of breeding little bustards Tetrax tetrax: ecological segregation based on vegetation structure. Acta Oecologica 34:345–353
Morales MB, Mañosa S, Villers A, de la Morena ELG, Bretagnolle V (2022) Migration, movements, and non-breeding ecology. In: Bretagnolle V, Traba J, Morales MB (eds) Little bustard: ecology and conservation. Springer, Cham, pp 123–150
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci 105:19052–19059
Nenzén HK, Araújo MB (2011) Choice of threshold alters projections of species range shifts under climate change. Ecol Modell 222:3346–3354
Neumann W, Martinuzzi S, Estes AB, Pidgeon AM, Dettki H, Ericsson G, Radeloff VC (2015) Opportunities for the application of advanced remotely-sensed data in ecological studies of terrestrial animal movement. Mov Ecol 3:8
Plante E, Vance R (1994) Selection of preschool language tests. Lang Speech Hear Serv Sch 25:15–24
Poggio L, de Sousa LM, Batjes NH, Heuvelink GBM, Kempen B, Ribeiro E, Rossiter D (2021) SoilGrids 2.0: producing soil information for the globe with quantified spatial uncertainty. Soil 7:217–240
Ponti R, Sannolo M (2022) The importance of including phenology when modelling species ecological niche. Ecography. https://doi.org/10.1111/ecog.06143
Rivas-Martínez S, Díaz González TE, Fernández-González F, Izco J, Loidi J, Lousã M, Penas A (2002) Vascular plant communities of Spain and Portugal. Itinera Geobotanica 15:5–432
Schoener TW (1968) The anolis lizards of bimini: resource partitioning in a complex fauna. Ecology 49:704–726
Silva JP, Pinto M, Palmeirim JM (2004) Managing landscapes for the little bustard Tetrax tetrax: lessons from the study of winter habitat selection. Biol Conserv 117:521–528
Silva JP, Faria N, Catry T (2007) Summer habitat selection and abundance of the threatened little bustard in Iberian agricultural landscapes. Biol Conserv 139:186–194
Silva JP, Palmeirim JM, Moreira F (2010) Higher breeding densities of the threatened little bustard Tetrax tetrax occur in larger grassland fields: implications for conservation. Biol Conserv 143:2553–2558
Silva JP, Estanque B, Moreira F, Palmeirim JM (2014) Population density and use of grasslands by female Little Bustards during lek attendance, nesting and brood-rearing. J Ornithol 155:53–63
Silva JP, Catry I, Palmeirim JM, Moreira F (2015) Freezing heat: thermally imposed constraints on the daily activity patterns of a free-ranging grassland bird. Ecosphere. https://doi.org/10.1890/ES14-00454.1
Silva JP, Moreira F, Palmeirim JM (2017) Spatial and temporal dynamics of lekking behaviour revealed by high-resolution GPS tracking. Anim Behav 129:197–204
Silva JP, Arroyo B, Marques AT, Morales MB, Devoucoux P, Mougeot F (2022) Threats affecting little bustards: human impacts. In: Bretagnolle V, Traba J, Morales MB (eds) Little bustard: ecology and conservation. Springer, Cham, pp 243–272
Smeraldo S, Di Febbraro M, Bosso L, Flaquer C, Guixé D, Lisón F, Meschede A, Juste J, Prüger J, Puig-Montserrat X, Russo D (2018) Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: lessons from bats. Biodivers Conserv 27:2425–2441
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293
Traba J, Morales MB (2019) The decline of farmland birds in Spain is strongly associated to the loss of fallowland. Sci Rep 9:1–6
Traba J, Sastre P, Morales MB (2013) Factors determining species richness and composition of steppe bird communities in Peninsular Spain: grass-steppe vs. shrub-steppe bird species. In: Morales MB, Traba J (eds) Steppe ecosystems. Biological diversity, management and restoration. NOVA Publishers, Hauppauge, pp 47–72
Traba J, Morales MB, Silva JP, Bretagnolle V, Devoucoux P (2022) Habitat selection and space use. In: Bretagnolle V, Traba J, Morales MB (eds) Little bustard: ecology and conservation. Springer, Cham, pp 101–122
European Union, Copernicus Land Monitoring Service (2021) https://land.copernicus.eu/ accessed 20 Oct 2021
Valerio F, Ferreira E, Godinho S, Pita R, Mira A, Fernandes N, Santos SM (2020) Predicting microhabitat suitability for an endangered small mammal using sentinel-2 data. Remote Sens (Basel) 12:562
Valerio F, Godinho S, Marques AT, Crispim-Mendes T, Pita R, Silva JP (2024) GEE_xtract: High-quality remote sensing data preparation and extraction for multiple spatio-temporal ecological scaling. Ecol Inform 80:102502. https://doi.org/10.1016/j.ecoinf.2024.102502
Valerio F, Godinho S, Salgueiro P, Medinas D, Manghi G, Mira A, Pedroso NM, Ferreira EM, Craveiro J, Costa P, Santos SM (2023) Integrating remote sensing data on habitat suitability and functional connectivity to inform multitaxa roadkill mitigation plans. Landsc Ecol. https://doi.org/10.1007/s10980-022-01587-6
von Keyserlingk J, de Hoop M, Mayor AG, Dekker SC, Rietkerk M, Foerster S (2021) Resilience of vegetation to drought: studying the effect of grazing in a Mediterranean rangeland using satellite time series. Remote Sens Environ 255:112270
Warren DL, Glor RE, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611
Wood EM, Pidgeon AM, Radeloff VC, Keuler NS (2012) Image texture as a remotely sensed measure of vegetation structure. Remote Sens Environ 121:516–526
Acknowledgements
We would like to thank all the colleagues, field technicians, and volunteers that have been participating in the capture and tagging campaigns of the Steppe Bird Move group since 2009. We also would like to acknowledge the Junta de Extremadura for the joint long-term tracking project of the little bustard. We are grateful to the editor and two anonymous reviewers for their valuable comments and suggestions to improve the paper.
Funding
Open access funding provided by FCT|FCCN (b-on). Work supported by the European Union’s Horizon 2020 Research and Innovation Programme under the Grant Agreement Number 857251. Bird tracking was funded by: EDP S.A. “Fundação para a Biodiversidade”; Movetech Telemetry; Enel, Green Power España, SL; EcoEnergías del Guadiana S.A. and NATURGY RENOVABLES, SLU. TCM was funded by National Funds through FCT - Foundation for Science and Technology under a doctoral grant (SFRH/BD/145156/2019). JPS was supported by an FCT contract (DL57/2019/CP1440/CT0021). RP was supported by the FCT thorough a research contract under the Portuguese Decree-Law nr 57/2016 and a CEEC research contract 2022.02878.CEECIND. SG was funded by the FUEL-SAT project “Integration of multi-source satellite data for wildland fuel mapping: the role of remote sensing for an effective wildfire fuel management” from Foundation for Science and Technology (PCIF/GRF/0116/2019), and by National Funds through FCT under the Project UIDB/05183/2020.
Author information
Authors and Affiliations
Contributions
All authors contributed critically to conceive the ideas and designed methodology; TCM, FV and ATM prepared the data; FV employed the modelling process; TCM analysed the data; TCM and JPS led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crispim-Mendes, T., Valerio, F., Marques, A.T. et al. High-resolution species distribution modelling reveals spatio-temporal variability of habitat suitability in a declining grassland bird. Landsc Ecol 39, 49 (2024). https://doi.org/10.1007/s10980-024-01848-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01848-6