Abstract
Context
The expansion of exotic plantations can impose conservation challenges on wildlife, and the Iberian Peninsula has one of the widest planted areas of exotic Eucalyptus sp. in Europe. Since mesocarnivores are pivotal elements of ecosystems’ functioning and Eucalyptus have been modifying the Portuguese landscape context in the last half century, it is crucial to understand how these systems may affect carnivores’ range.
Objectives
We aim to identify the drivers of five mesocarnivores’ distribution in Portugal (e.g., land-cover, ecogeographic predictors, mammal prey availability) and understand the influence of Eucalyptus plantations in their distribution range.
Methods
Using generalized linear models, we modelled the distribution range of mesocarnivores. The initial dataset was randomly split for model training and validation, and the multicollinearity between the predictors was tested. Then, we examined the potential relationship between the Eucalyptus plantations area and the predicted probability presence of each species.
Results
We detected species-specific patterns explained by different drivers, including climatic, land cover and mammal prey related ones. Furthermore, in areas of Eucalyptus plantations, the probability of occurrence of most Portuguese mesocarnivores is lower: red fox,stone marten,European badger, and Egyptian mongoose.
Conclusions
Managers must take action to adapt their management to promote native forest patches within plantation, and allow the development of some understory within stands, to improve this plantation’s permeability to mesocarnivores. This will increase the spatial heterogeneity and enhance resource availability, reducing the constraints that plantations might have on the range of mesocarnivores in Portugal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mediterranean Basin is a crossroad of species of African and European origins (Critical Ecosystem Partnership Fund 2017), which together with a historical human presence that shaped the region’s environmental conditions, resulted in one of the most important biodiversity hotspots (Myers et al. 2000; Critical Ecosystem Partnership Fund 2017). Within the Basin, the Iberian Peninsula is a pivotal wildlife conservation area, due to the interaction between biodiversity values and historical landscapes composed, for instance, by Dehesas (Spanish name) or Montados (Portuguese name) (Maranon 1988). But in many areas the increasing human demand for wood and paper pulp has led to the replacement of the native (e.g., shrublands) and some of those historical habitats (e.g., woodlands) by plantations, especially Eucalyptus sp. (Deus et al. 2018; DGT 2020; FAO 2020).
The introduction of Eucalyptus in the Iberian Peninsula dates to the late 19th century and the area covered has been expanding ever since, with an increasing rate since the 1960s (FAO 1981; Alves et al. 2007; Veiras and Soto 2011; Deus et al. 2018). This exotic species, originally from Australia, can pose a threat to the conservation of wildlife species that have long been co-existing with, and adapting to, the traditional ecosystems of Iberia, especially if occurring as homogeneous and intensive plantations (da Silva et al. 2019). Various studies have highlighted the impacts of these plantations on local communities, inducing lower vertebrate diversity and abundance, often linked to limited food resources that can be used by these species (Rosalino and Santos-Reis 2009; Zahn et al. 2010; Calviño-Cancela et al. 2012; Martin et al. 2012; Teixeira et al. 2017; da Silva et al. 2019), or less shelter and protective cover (Teixeira et al. 2017; da Silva et al. 2019). Although this pattern is common in many areas where Eucalyptus has been established as a forestry species, there are only a few studies in Iberia, and particularly in Portugal, that have targeted the effect of Eucalyptus on the carnivore communities (but see Cruz et al. 2015; Teixeira et al. 2023), some of which are species-specific (e.g., Castro et al. 2022).
Assessing how the implementation of Eucalyptus plantations can affect the carnivores’ guild is pivotal for contributing to the maintenance of Iberia’s natural heritage. Carnivores are core ecosystem elements that maintain biome functionality (Mangas et al. 2008; Roemer et al. 2009), landscape structure, and resilience (Roemer et al. 2009), due to, among others, their role as seed dispersal (Rosalino et al. 2010) and prey density controllers, i.e., small mammals (Salo et al. 2010; Williams et al. 2018). In altered landscapes, large or apex predators are often absent (Teixeira et al. 2020) as they are more sensitive to human pressure, disturbance, and habitat fragmentation and loss (Laliberte and Ripple 2004; Prugh et al. 2009). Inversely, mesocarnivores—mid-sized carnivore species with less than 15 kg (Roemer et al. 2009)—are much more diverse in their behavior and ecology, being often generalists and more resilient than apex species, reaching higher species richness and abundance in man-shaped environments when compared to large carnivores (Roemer et al. 2009). Owing to their smaller size and the ability to prosper in distinct habitats by using different resources, they often use landscapes that are shaped by humans and their activities (Alexandre et al. 2020). However, many of them are also less frequently the target of scientific studies in anthropic landscapes, such as plantations (but see Cruz et al. 2015; Bencatel et al. 2018), which constrain our ability to understand the effect of landscape changes on this group (see Márquez et al. 2022). Our ability to understand their ecological strategies to cope with the conservation challenges imposed by exotic plantations and man-related systems is therefore limited.
In Portugal, there are 15 species of carnivores, 13 of which are considered mesocarnivores (Álvares et al. 2019). Since not all mesocarnivores are widely distributed in mainland Portugal, we focused this study on the five terrestrial mesocarnivores with wider distribution ranges according to Álvares et al. (2019)—Vulpes vulpes—red fox; Martes foina—stone marten; Genetta genetta—common genet; Meles meles—European badger; and Herpestes ichneumon—Egyptian mongoose. Our main goal is to understand if the Eucalyptus plantations influence the distribution range of the most representative mesocarnivore community in continental Portugal. Complementarily, we also aimed to identify other drivers (e.g., land cover, climate, mammal prey presence and/or richness, among others) that may affect the species distribution. We hypothesize that Eucalyptus plantations will globally constrain the distribution range of mesocarnivores in Portugal continental (Cruz et al. 2015) since these areas are usually poor providers of food and shelter for these species (Mangas et al. 2008; Ramírez and Simonetti 2011).
Methods
Study area
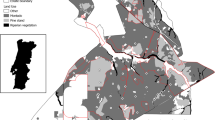
Portugal is the most Southwestern country of the Mediterranean Basin, with an area of 88,889 Km2. The region’s climatic conditions are characterized by humid cold winters and hot dry summers, and its landscape profoundly affects vegetation and wildlife (European Commission et al. 2010). Its geographical isolation and the great diversity of habitats and biotopes have allowed the development of its characteristic flora and fauna, including many endemic taxa (Myers et al. 2000; Pascual et al. 2011). The most representative land covers in the country are forests, accounting for 39% of the Portuguese territory, including native forests (e.g., Quercus deciduous forest, pine forest), high conservation value agroforestry systems (e.g., “montado” forests of Quercus suber and Q. ilex; DGT 2020) and exotic plantations. Of the entire forested area of Portugal, Eucalyptus plantations account for 27% (DGT 2020) (Fig. 1). From 2015 to 2018, the area of plantations of this exotic tree increased by 83,000 hectares, totalizing, currently, 928,000 hectares nationwide (ICNF 2015; DGT 2020).
Eucalyptus plantation evolution since 1995, 2007, and until 2018 using data collected from Land Use and Occupancy Map of Continental Portugal (COS 2018) available for download at https://snig.dgterritorio.gov.pt/rndg/srv/eng/catalog.search#/metadata/b498e89c-1093-4793-ad22-63516062891b
Sampling scale and data collection
Continental Portugal was divided into a UTM grid of 10 × 10 km (N = 1005 territorial units). We then extracted the presence records of five mesocarnivores (red fox, stone marten, common genet, European badger, and Egyptian mongoose) from the “Mammal Atlas of Portugal” (https://atlasmamiferosportugal.wordpress.com/; Bencatel et al. 2019). We only collected records labeled as ‘confirmed’, including all unequivocal records, such as direct observation or capture of live animals, dead animals, specimen photos, and genetically identified samples (Bencatel et al. 2019). The collected presence records of all species are dated until the end of 2018 (see Figure A.1—presence map for each species; Appendix A).
Drivers of carnivores’ distribution
To identify the drivers of mesocarnivores’ distribution range in continental Portugal, we calibrated spatially explicit species distribution models based on ecogeographical predictors (EGVs), namely climatic, topographic and land use-related variables (Rahbek et al. 2007). We tested 38 EGVs (climatic, land use, topographic, and anthropical variable) based on their potential capability to explain the occurrence of the species targeted in this study (Martínez et al. 2022; Torre et al. 2022) (see Table B.1 Appendix B). In addition, we estimated four prey species presence and richness-related variables [European rabbit (Oryctolagus cuniculus), Iberian hare (Lepus granatensis), rodent richness, and insectivorous mammals’ richness] in each territorial unit of 10 × 10 km, using the data collected by Bencatel et al. (2019), as prey availability may constrain mesocarnivores occurrence (Foster et al. 2013; Vilella et al. 2020).
All the information collected for the different variables listed in Table B1; Appendix B, estimated for each 10 × 10 km cell, was processed using the software QGIS, version 3.10.8 (QGIS Development Team 2020).
Data analysis
The dataset was randomly split into 70–30% subsets for model training and validation, respectively. To avoid multicollinearity between the numerical predictors, we assessed the predictor’s variance inflation factor (VIF). The VIFs were analyzed using the Heiberger method (Heiberger 2012) with R package ‘usdm’ (Naimi et al. 2014) to find a set of predictors without collinearity (i.e., VIF < 3; Zuur et al. 2010). All non-collinear variables (see Table 1) were finally used to parameterize Generalized Linear Models (GLMs) for explaining species distribution range (one model for each species), using binomial distribution and a logistic link function (Cameron and Trivedi 2013). All the continuous variables were standardized. The best models for each species were produced on the training datasets by using a forward-backward stepwise procedure, based on Akaike’s information criteria (AIC) (Burnham and Anderson 2002). We calculated the difAIC, i.e., the difference between the AIC of each species null model and the AIC of produced models including drivers, to access the models’ usefulness (i.e., when difAIC > 2). As GLMs are based in presence/absence data and data for carnivore presence can be incomplete due to the low detectability of this group of species, we also performed species distribution models using presence-background Maxent models (Phillips et al. 2006). We conducted a Spearman correlation analysis between the predicted values generated by the Maxent and Generalized Linear models, to assess the agreement of both analytical methods and thus the robustness of our results. Maxent results were summarized in Appendix D. As a strong concordance was obtained in the predicted patterns from GLMs and Maxent (Figure D.1 and Table D.1; Appendix D; strong correlations - > 0.8 - across all species), subsequent analyses were carried out only with the GLMs results.
We assessed the models’ predictive performance on the validation datasets by estimating the area under the ROC curve (AUC). According to Manel et al. (2001), AUC values between 0.5 and 0.7 indicate low accuracy, while models with values between 0.7 and 0.9 can predict species presence accurately and AUC > 0.9 indicates that models have high accuracy. The AUC value was computed using the ‘ROCR’ R package (Sing et al. 2005). The reliability of the predicted probabilities was assessed by exploring the calibration plots based on validation datasets (Pearce and Ferrier 2000). We plotted the observed frequency of each species’ presence against the predicted probability of presence, using the R package ‘ggplot2’ (Wickham 2016). Once the models were validated, they were projected to the full extent of continental Portugal, using the R package ‘fuzzySim’ (Barbosa 2015).
In this first phase of the analysis (Fig. 2), we excluded the Eucalyptus plantation cover area from the independent variable’s set. Our main goal was to test if carnivores’ range could be constrained by Eucalyptus cover, but the higher importance of other predictors as determinants of carnivore’s range can mask the detection of the effect of plantations’ cover (i.e., the spatial pattern of Eucalyptus could be explained by a combination of EGV that could dilute or even mask its potential relationship with the target species). Thus, we opted to first produce good-fitting models, based on the most commonly identified environmental drivers (described in Table 1), and in a second phase (Fig. 2), assess if there was a relation between the predicted probability of presence and the Eucalyptus proportion of cover. Thus, we performed a linear regression for each species’ predicted presence probability and the proportion of area covered by Eucalyptus plantations in each 10 × 10 km square. We assessed if Eucalyptus plantations cover predictor could account for some of the probability of species occurrence variation, by comparing the AIC of the null model (without predictors), with that of the model that included the Eucalyptus cover proportion. We estimated the difAIC, but this time we used the difference between the AIC of the null models and that of produced models including Eucalyptus cover, for each species, and considered that there is very high or high support for the potential relation of Eucalyptus land cover proportion in driving species distribution range when difAIC > 10 or difAIC > 6, respectively (Burnham and Anderson 2002). The statistical analyses were carried out in R 4.0.5 (R Core Team 2021).
Results
All selected species (red fox, stone marten, common genet, European badger and Egyptian mongoose), showed a presence record covering most of continental Portugal. Nevertheless, Egyptian mongoose and stone marten were not detected in Northwestern and in most of the coastline respectively (Figure A.1; Appendix A).
From the initial set of predictors, 25 were removed from the analysis due to collinearity problems (i.e., VIF > 3; see Table B.1; Appendix B). The remaining 13 EGVs and the four prey variables listed in Table 1, were used in the subsequent models. The most parsimonious model for each species is summarized in Table 2.
Globally, the presence of both lagomorphs (i.e., European rabbit and Iberian hare) has a positive influence on the mesocarnivores distribution and were the most influential variables in the majority of the analyzed species. However, there are some specific variations, with species presence probability being significantly higher in areas with higher slope (red fox and stone marten), mean annual potential evapotranspiration (annualPET; stone marten), precipitation seasonality (‘Bio 15’; Egyptian mongoose), lower scrub (‘X6_Scrub’; European badger and common genet), and anthropic land cover (‘X1_Territ’; Egyptian mongoose and European badger). All best models’ AUC values were > 0.7, indicating their capability to accurately predicted the presence of species (Table 3). The predicted occurrence probabilities for each mesocarnivore species are present in Fig. 3, and the calibration plots of the observed frequency of each species’ presence against the predicted probability of presence are represented in Figure C.1 (Appendix C).
The AIC values obtained for the models produced for every species were lower than that of the null models. The difAIC was always > 30, indicating that those models had high support when compared to the null models (Table 3).
The surface of Eucalyptus plantations within which 10 × 10 km squares was negatively related to four mesocarnivores’ probability occurrence (Table 4). Only for common genets this pattern was not detected (Table 4).
Discussion
Influence of Eucalyptus plantations on mesocarnivores distribution
Eucalyptus plantations have been established in several European countries, but Portugal and Spain concentrate most of the European Eucalyptus plantations (Tomé et al. 2021). It is assumed that the high landscape proportion covered by these exotic plantations in Iberia would induce changes in the native communities, due to changes in resource availability, and several studies’ results have corroborated this expected pattern (revised by Tomé et al. 2021). Nevertheless, most are of local/regional scope, and few targeted mesocarnivores (e.g., Cruz et al. 2015; Castro et al. 2022). Our national scale study on mesocarnivores fills the wide range study gap.
All mesocarnivores tested in our analyses seem to occur in distinct types of habitats, from anthropic landscapes, such as agroforest areas, to native forest habitats, emphasizing their ability to explore different habitats. Nevertheless, the presence of Eucalyptus plantations is consistently related to mesocarnivores distribution in Portugal, as areas with a higher cover of Eucalyptus plantations tend to have a lower presence probability by most species.
These constraints caused by the presence of plantations of this exotic tree can derive from the landscape transformation occurring when establishing plantations. Most Eucalyptus plantations, cultivated mainly for pulpwood production (Alves et al. 2018) are monospecific, harvested in 10–12 years rotation cycles (Alves et al. 2007; Silva and Tomé 2013), and show limited understory strata (da Silva et al. 2019). Such structure limits the type, diversity, and quantity of resources (e.g., prey species) they can provide for mesocarnivores (Carrilho et al. 2017; Teixeira et al. 2017), which may limit their use by these mammalian predators. But mesocarnivores’ distribution in Eucalyptus plantations may not only be limited by the presence of prey (da Silva et al. 2019) but also by different factors such as refuge availability (e.g., lack of understory; Timo et al. 2014), species locomotion mode (e.g., arboreal species; Ferreira et al. 2018), disturbance scale (e.g., human activities; e.g., Castro et al. 2022), among others. All these factors depend on the type and intensity of management that will determine the stand structure (amount of understory available, presence of native trees inside stands that allows arboreal species to move to the canopy, etc.) and disturbance spatial and temporal scale (i.e., where, when and for how long will forestry workers be present within plantations and what kind of machinery will be used and when). Some authors have found a notorious preference for native and resource-rich habitats by badgers (Revilla et al. 2000; Rosalino et al. 2004, 2008; Cruz et al. 2015) as they select those habitat types due to key resources, such as food and shelter (Revilla et al. 2000; Rosalino et al. 2004). Stone martens also prefer old-growth mixed woodland and evergreen oak forest, where food and refuges are more accessible, avoiding Eucalyptus plantations (Pereira et al. 2012; Cruz et al. 2015). This pattern is also observed at the macroecological scale of this study. Mesocarnivores’ distribution ranges (Figure A.1 Appendix A) showed a clear lack of stone marten presence records along the entire Portuguese coastal zone, where there is a predominance of Eucalyptus plantations (Fig. 1). But this is probably not the only driver limiting stone martens’ distribution, since the species is also absent in areas where Eucalyptus plantations are not present. Other Mediterranean populations are absent or less common in areas with a high proportion of scrublands, croplands, and urban areas (e.g., Vergara et al. 2016). However, we were unable to detect a deleterious effect of these drivers on the Portuguese stone marten population. Therefore, other not accounted drivers (e.g., human density; Balestrieri et al. 2019) may be contributing to the detected pattern.
Regarding species locomotion mode, common genet has arboreal locomotion and isn’t affected by Eucalyptus occupancy. Genets tend to prefer oak forests, often selecting Quercus rotundifolia, Q. suber, and Arbutus unedo woodlands (Zuberogoitia et al. 2002; Sarmento et al. 2010; Carvalho et al. 2014). They may avoid scrubland areas with specific composition (e.g., Erica spp. and Cistus ladanifer) and Eucalyptus stands (Sarmento et al. 2010). However, when Eucalyptus plantations include dense bramble understory (e.g., Rubus spp.) genets may still use these areas to avoid potential predators (e.g., dogs and cats; Zuberogoitia et al. 2002), as these patches provide an efficient refuge or competitors that may avoid plantations (e.g. foxes; Castro et al. 2022; Santos et al. 2007). This species also tends to reduce its presence in areas with frequent human disturbance (i.e., near houses and roads; Espírito-Santo et al. 2007).
Other drivers of mesocarnivores’ distribution range
A myriad of other factors related to prey availability, distribution, climate, and land use contribute, isolated or in synergy, determine mesocarnivores’ distribution in Portugal (Hipólito et al. 2018; Rosalino et al. 2019; Alexandre et al. 2020). Our data indicate that prey-related drivers can facilitate species presence in Portugal; concretely lagomorph presence, promote all five mesocarnivores’ presence. European rabbit and Iberian hare are known as important food sources for many carnivores (Carvalho and Gomes 2001; Rosalino and Santos-Reis 2002; Sillero-Zubiri et al. 2004; Rosalino et al. 2009b; Verdade et al. 2011; Díaz-Ruiz et al. 2013). Therefore, their presence adds food resources to the landscape that can be used by mesocarnivores, allowing them to inhabit regions where those prey subsist.
Inversely, human presence can restrain wildlife’s distribution range (Oberosler et al. 2017), due to an increase in disturbance (including active persecution), changes in landscape structure, and available resources (Reason et al. 1993; Frick et al. 2020). Anthropic land uses (e.g., buildings, infrastructure, road networks, transportation, among others) can negatively influence some mesocarnivores (e.g., Egyptian mongoose and European badger) due to the increased disturbance they induce (Prigioni and Deflorian 2005; Barros et al. 2015). Predators may also minimize this disturbance effect by avoiding areas where humans are most common (e.g., plain or low slopes areas; Plate 2006), and using regions with higher slopes (e.g., in Canada, red foxes use the ravine slopes significantly more than expected since slopes were rarely used by humans; Adkins and Stott 1998). Two of the modelized species (red fox and stone marten) showed a preference for stepper areas, corroborating this avoidance behavior towards areas more used by humans.
Only two bioclimatic drivers, the precipitation seasonality, and the mean annual potential evapotranspiration, were influential in the distribution of species. Precipitation seasonality had a positive influence on Egyptian mongoose distribution. This species, whose core range is in Africa (Delibes 1999) is more likely to occur in areas that have a greater precipitation variation between winter and summer, such as in southern Portugal, where summer is very dry (and the driest period is longer), and winter is wily wet, a typically Mediterranean climate. This effect is corroborated by the fact that there are practically no records of this species in the northwest of the country, a region with a typically Atlantic climate, where precipitation seasonality is lower (Álvares et al. 2019). The other important climatic driver, which affects stone marten, was evapotranspiration, which corresponds to the amount of water loss from evaporation as well as transpiration and is related to plant productivity. In areas with higher evapotranspiration, there is an increase in plant productivity. Therefore, food production in such regions is usually higher, promoting food availability for mesocarnivores (e.g., berries and fruit—important items in Mediterranean stone marten diet–Barrull et al. 2014; Lima 2021).
According to different studies, the landscape composition can be a pivotal driver of mesocarnivores’ distribution. Scrublands can promote mesocarnivores’ presence [e.g., genets (Virgós and Casanovas 1997), and badgers (Revilla et al. 2001)], by providing broader resource availability (e.g., food availability and shelter - Mangas et al. 2008; Carrilho et al. 2017). However, the influence of shrub cover depends on how well-developed and dense the understory is (Curveira-Santos et al. 2017), or the representativeness of another land cover, such as forest (Alexandre et al. 2020). For badgers, genets, and foxes, scrub areas had a negative influence on their distribution, although with a small effect (i.e., low coefficient values). We think that the lower food resources present in Mediterranean shrublands (when compared to other covers like riparian areas—Rosalino et al. 2009a) may overrule the shelter opportunities this land cover provides (Mangas et al. 2008).
Implications for Eucalyptus plantations management
Most species are usually more abundant, and the overall species richness is higher, in native forests when compared with plantations (Bremer and Farley 2010; Brockerhoff et al. 2013). But this overall pattern can vary, depending on the plantation’s landscape structure and composition. In plantations where the canopies are not contiguous, a greater amount of light can reach the ground, thus promoting the development of the understory vegetation (i.e., herbaceous and shrub layers), leading to higher prey abundances (Teixeira et al. 2017), which in turn will favor predators’ presence (e.g., mesocarnivores). Some studies even show that Eucalyptus plantations with dense or complex understories can support species with densities and/or occurrence probabilities similar to those found in native forests (Fogarty and Vilella 2003; Silva-Rodríguez and Sieving 2012). These patterns show that management options can really make a difference in promoting regional biodiversity. Since natural areas are known to provide more resources and harbor a higher abundance of species (Torre et al. 2022), forestry managers need to prioritize the preservation of other habitats (e.g., riparian galleries) within Eucalyptus plantations, as they are key habitats to some carnivores (Gehring and Swihart 2003; Mestre et al. 2007; Matos et al. 2009). Patches of other habitats or linear structures (e.g., riparian forests, hedgerows, streams) within Eucalyptus, will allow the homogeneity of the plantations to be broken, add resources usable by wildlife, and can act as corridors for the species movements (Cruz et al. 2015), which altogether will facilitate mesocarnivore’ presence and use of plantations, mitigating its possible barrier effect (Kupfer et al. 2006).
Conclusion
It is accepted that Eucalyptus plantations support lower levels of biodiversity than natural mixed forests (Brockerhoff et al. 2013), and several studies conducted in the Iberian Peninsula corroborated this pattern (see Tomé et al. 2021 for a summary of studies). Our study appears to follow similar reasoning by indicating that the distribution of most mesocarnivores species seems to be constrained by Eucalyptus plantations. However, it is not only the presence of this exotic plantation that influences mesocarnivores’ distribution patterns. Other factors also contribute to the detected pattern, namely variables associated with prey availability, clime, other land use, and topography. Therefore, Eucalyptus managers need to consider the biodiversity dimension when defining their management plans and thus should adopt a strategy that minimizes the detected effect of plantations on mesocarnivores distribution, to assure that production is done in the most sustainable way possible and are not another biodiversity loss driver.
Data availability
The datasets used during the current study are available at “Mammal Atlas of Portugal” (https://atlasmamiferosportugal.wordpress.com/; Bencatel et al. 2018).
References
Adkins CA, Stott P (1998) Home ranges, movements and habitat associations of red foxes Vulpes vulpes in suburban Toronto, Ontario, Canada. J Zool 244:335–346.
Alexandre M, Hipólito D, Ferreira E, Fonseca C, Rosalino LM (2020) Humans do matter: determinants of red fox (Vulpes vulpes) presence in a Western Mediterranean landscape. Mammal Res 65:203–214.
Álvares F, Ferreira CC, Barbosa AM, Rosalino LM, Pedroso NM, Bencatel J (2019) Carnívoros. In: Bencatel J, Sabino-Marques H, Álvares F, Moura A, Barbosa AM (eds) Atlas de mamíferos de Portugal, 2a edição. p 274
Alves AM, Pereira JS, Silva JMN (2007) O Eucaliptal em Portugal: Impactes Ambientais e Investigação Científica. ISAPress, Lisbon
Alves AM, Almeida MH, Goes A (2018) Plantações florestais. ISAPress, Lisbon
Balestrieri A, Mori E, Menchetti M, Ruiz-González A, Milanesi P (2019) Far from the madding crowd: tolerance toward human disturbance shapes distribution and connectivity patterns of closely related Martes spp. Popul Ecol 61:289–299.
Barbosa AM (2015) fuzzySim: applying fuzzy logic to binary similarity indices in ecology. Methods Ecol Evol 6:853–858.
Barros T, Carvalho J, Pereira MJR, Ferreira JP, Fonseca C (2015) Following the trail: factors underlying the sudden expansion of the egyptian mongoose (Herpestes ichneumon) in Portugal. PLoS ONE 10:1–18.
Barrull J, Mate I, Ruiz-Olmo J, Casanovas JG, Gosàlbez J, Salicrú M (2014) Factors and mechanisms that explain coexistence in a Mediterranean carnivore assemblage: an integrated study based on camera trapping and diet. Mamm Biol 79:123–131.
Bencatel J, Ferreira CC, Barbosa M, Rosalino LM, Álvares F (2018) Research trends and geographical distribution of mammalian carnivores in Portugal (SW Europe). PLoS ONE 13:1–20.
Bencatel J, Sabino-Marques H, Álvares F, Moura A, Barbosa AM (2019) Atlas de mamíferos de Portugal. Universidade de Évora, Évora
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915.
Brockerhoff EG, Jactel H, Parrotta JA, Ferraz SFB (2013) Role of eucalypt and other planted forests in biodiversity conservation and the provision of biodiversity-related ecosystem services. Ecol Manag 301:43–50.
Burnham KP, Anderson DR (2002) Model selection and Multimodel Inference: a practical information-theoretic approach. Springer-Verlag, New York
Calviño-Cancela M, Rubido-Bará M, van Etten EJB (2012) Do eucalypt plantations provide habitat for native forest biodiversity? Ecol Manag 270:153–162.
Cameron AC, Trivedi PK (2013) Regression analysis of count data, 2nd edn. Cambridge University Press, New York
Carrilho M, Teixeira D, Santos-Reis M, Rosalino LM (2017) Small mammal abundance in Mediterranean Eucalyptus plantations: how shrub cover can really make a difference. Ecol Manag 391:256–263.
Carvalho JC, Gomes P (2001) Food habits and trophic niche overlap of the red fox, European wild cat and common genet in the Peneda-Gerês National Park. Galemys 13:39–48
Carvalho F, Carvalho R, Mira A, Beja P (2014) Use of tree hollows by a Mediterranean forest carnivore. Ecol Manag 315:54–62.
Castro G, Teixeira D, Ares-Pereira G, Lima C, Magalhães A, Camarinha C, Guillera-Arroita G, Fonseca C, Rosalino LM (2022) Drivers of occupancy patterns for the red fox, Vulpes vulpes, in Mediterranean Eucalyptus plantations. For Ecol Manage. https://doi.org/10.1016/j.foreco.2022.120293
R Core Team (2021) A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
COS (2018) Carta de uso e ocupação do solo de Portugal continental para 2018. Direção-Geral do Território, Lisboa.
Critical Ecosystem Partnership Fund (2017) Ecosystem profile - Mediterranean Basin biodiversity hotspot. Conservation International, Washington D.C.
Cruz J, Sarmento P, White P (2015) Influence of exotic forest plantations on occupancy and co-occurrence patterns in a Mediterranean carnivore guild. J Mammal 96:854–865.
Curveira-Santos G, Marques TA, Björklund M, Santos-Reis M (2017) Mediterranean mesocarnivores in spatially structured managed landscapes: community organisation in time and space. Agric Ecosyst Environ 237:280–289.
da Silva L, Heleno RH, Costa JM, Valente M, Mata VA, Gonçalves SC, da Silva A, Alves J, Ramos JA (2019) Natural woodlands hold more diverse, abundant, and unique biota than novel anthropogenic forests: a multi-group assessment. Eur J for Res 138:461–472.
Delibes M (1999) Herpestes ichneumon. In: Mitchell-Jones AJ, Amori G, Bogdanowicz W, Kryštufek B, Reijnders PJH, Spitzenberger F, Stubbe M, Thissen JBM, Vohralík V, Zima J (eds) The Atlas of European Mammals. Academic Press, London, UK, pp 356–357
Deus E, Silva JS, Castro-Díez P, Lomba A, Ortiz ML, Vicente J (2018) Current and future conflicts between eucalypt plantations and high biodiversity areas in the Iberian Peninsula. J Nat Conserv 45:107–117.
DGT (2020) Uso E Ocupação Do Solo Em Portugal Continental - Análises Temáticas 1. Direção-Geral do Território, Lisboa.
Díaz-Ruiz F, Delibes-Mateos M, García-Moreno JL, María López-Martín J, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mamm Rev 43:59–70.
Espírito-Santo C, Rosalino LM, Santos-Reis M (2007) Factors affecting the placement of common genet latrine sites in a Mediterranean landscape in Portugal. J Mammal 88:201–207.
European Commission, Directorate-General for Environment, Sundseth K (2010) Natura 2000 in the Mediterranean region. European Commission, Bruxels.
FAO (1981) Los eucaliptos como árboles en plantaciones. In: FAO (ed) El eucalipto en la repoblacion forestal. FAO, Rome.
FAO (2020) Global Forest Resources Assessment 2020: Main report. FAO, Rome
Ferreira AS, Peres CA, Bogoni JA, Cassano CR (2018) Use of agroecosystem matrix habitats by mammalian carnivores (Carnivora): a global-scale analysis. Mamm Rev 48:312–327.
Fogarty JH, Vilella FJ (2003) Use of native forest and Eucalyptus Plantations by Eleutherodactylus Frogs. Source J Wildl Manag 67:186–195
Foster VC, Sarmento P, Sollmann R, Tôrres N, Jácomo ATA, Negrões N, Fonseca C, Silveira L (2013) Jaguar and Puma activity patterns and predator-prey interactions in four brazilian biomes. Biotropica 45:373–379.
Frick WF, Kingston T, Flanders J (2020) A review of the major threats and challenges to global bat conservation. Ann N Y Acad Sci 1469:5–25.
Gehring TM, Swihart RK (2003) Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: mammalian predators in an agricultural landscape. Biol Conserv 109:283–295.
Heiberger RM (2012) HH: Statistical Analysis and Data Display: Heiberger and Holland R package version 2.3–27
Hipólito D, Guedes D, Cabecinha D, Serronha A, Grilo C, Santos-Reis M, Monterroso P, Carvalho J, Fonseca C, Pardavila X, Virgós E, Rosalino LM (2018) Drivers of sett site location by european badgers in Portugal. Biodivers Conserv 27:2951–2970.
ICNF (2015) 6o Inventário Florestal Nacional. Relatório Final, ICNF, Lisboa.
Kupfer JA, Malanson GP, Franklin SB (2006) Not seeing the ocean for the islands: the mediating influence of matrix-based processes on forest fragmentation effects. Glob Ecol Biogeogr 15:8–20.
Laliberte AS, Ripple WJ (2004) Range Contractions of North American Carnivores and Ungulates. Bioscience 54:123–138
Lima CAS (2021) Mesocarnivore trophic relationships in Mediterranean landscapes: the case study of red fox and stone marten. University of Aveiro, Aveiro
Manel S, Williams HC, Ormerod SJ (2001) Evaluating presence-absence models in ecology: the need to account for prevalence. J Appl Ecol 38:921–931.
Mangas JG, Lozano J, Cabezas-Díaz S, Virgós E (2008) The priority value of scrubland habitats for carnivore conservation in Mediterranean ecosystems. Biodivers Conserv 17:43–51.
Maranon T (1988) Agro-sylvo-pastoral systems in the Iberian Peninsula: dehesas and montados. Rangelands Archives 10:255–258
Márquez C, Ferreira CC, Acevedo P (2022) Driver interactions lead changes in the distribution of imperiled terrestrial carnivores. Sci Total Environ 838:156165.
Martin PS, Gheler-costa C, Lopes PC, Rosalino LM, Verdade LM (2012) Terrestrial non-volant small mammals in agro-silvicultural landscapes of Southeastern Brazil. For Ecol Manage 282:185–195. https://doi.org/https://doi.org/10.1016/j. foreco.2012.07.002
Martínez JIZ, Seoane J, Kelly MJ, Sarasola JH, Travaini A (2022) Assessing carnivore spatial co-occurrence and temporal overlap in the face of human interference in a semiarid forest. Ecol Appl 32(1):e02482.
Matos HM, Santos MJ, Palomares F, Santos-Reis M (2009) Does riparian habitat condition influence mammalian carnivore abundance in Mediterranean ecosystems? Biodivers Conserv 18:373–386.
Mestre FM, Ferreira JP, Mira A (2007) Modelling the distribution of the european Polecat Mustela putorius in a mediterranean agricultural landscape. Rev d’Ecologie, Terre Vie, Société Natl Prot la Nat 62:35–47
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37:191–203.
Oberosler V, Groff C, Iemma A, Pedrini P, Rovero F (2017) The influence of human disturbance on occupancy and activity patterns of mammals in the italian Alps from systematic camera trapping. Mamm Biol 87:50–61.
Pascual L-L, Luigi M, Alessandra F, Emilio B, Luigi B (2011) Hotspots of species richness, threat and endemism for terrestrial vertebrates in SW Europe. Acta Oecol 37:399–412.
Pearce J, Ferrier S (2000) An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol Modell 128:127–147.
Pereira P, da Silva A, Alves J, Matos M, Fonseca C (2012) Coexistence of carnivores in a heterogeneous landscape: Habitat selection and ecological niches. Ecol Res 27:745–753.
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259.
Plate EJ (2006) Disaster Prevention. In: Ehlers E, Krafft T (eds) Earth System Science in the Anthropocene. Springer, Berlin, Heidelberg, p 273
Prigioni C, Deflorian MC (2005) Sett site selection by eurasian badger (Meles meles) in an italian Alpine area. Ital J Zool 72:43–48.
Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS (2009) The rise of the Mesopredator. Bioscience 59:779–791.
QGIS Development Team (2020) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
Radosavljevic A, Anderson RP (2014) Making better Maxent models of species distributions: complexity, overfitting and evaluation. J Biogeogr 41:629–643.
Rahbek C, Gotelli NJ, Colwell RK, Entsminger GL, Rangel TFLVB, Graves GR (2007) Predicting continental-scale patterns of bird species richness with spatially explicit models. Proc R Soc B Biol Sci 274:165.
Ramírez PA, Simonetti JA (2011) Conservation opportunities in commercial plantations: the case of mammals. J Nat Conserv 19:351–355.
Reason P, Harris S, Cresswell P (1993) Estimating the impact of past persecution and habitat changes on the numbers of Badgers Meles meles in Britain. Mamm Rev 23:1–15.
Revilla E, Palomares F, Delibes M (2000) Defining key habitats for low density populations of eurasian badgers in Mediterranean environments. Biol Conserv 95:269–277.
Revilla E, Palomares F, Fernández N (2001) Characteristics, location and selection of diurnal resting dens by eurasian badgers (Meles meles) in a low density area. J Zool 255:291–299.
Roemer GW, Gompper ME, Valkenburgh B, Van (2009) The ecological role of the mammalian mesocarnivore. Bioscience 59:165–173.
Rosalino LM, Santos-Reis M (2002) Feeding habits of the common genet Genetta genetta (Carnivora: Viverridae) in a semi-natural landscape of central Portugal. Mammalia 66:195–205.
Rosalino LM, Santos-Reis M (2009) Fruit consumption by carnivores in Mediterranean Europe. Mamm Rev 39:67–78.
Rosalino LM, Macdonald DW, Santos-Reis M (2004) Spatial structure and land-cover use in a low-density Mediterranean population of eurasian badgers. Can J Zool 82:1493–1502.
Rosalino LM, Santos MJ, Beier P, Santos-Reis M (2008) Eurasian badger habitat selection in Mediterranean environments: does scale really matter? Mamm Biol 73:189–198.
Rosalino LM, Rosário J, do, Santos-Reis M (2009a) The role of habitat patches on mammalian diversity in cork oak agroforestry systems. Acta Oecol 35:507–512.
Rosalino LM, Santos MJ, Pereira I, Santos-Reis M (2009b) Sex-driven differences in egyptian mongoose’s (Herpestes ichneumon) diet in its Northwestern european range. Eur J Wildl Res 55:293–299.
Rosalino LM, Rosa S, Santos-Reis M (2010) The role of carnivores as Mediterranean seed dispersers. Ann Zool Fenn 47:195–205
Rosalino LM, Guedes D, Cabecinha D, Serronha A, Grilo C, Santos-Reis M, Monterroso P, Carvalho J, Fonseca C, Pardavila X, Virgós E, Hipólito D (2019) Climate and landscape changes as driving forces for future range shift in southern populations of the european badger. Sci Rep 9:1–15.
Salo P, Banks PB, Dickman CR, Korpimäki E (2010) Predator manipulation experiments: impacts on populations of terrestrial vertebrate prey. Ecol Monogr 80:531–546.
Santos MJ, Pinto BM, Santos-Reis M (2007) Trophic niche partitioning between two native and two exotic carnivores in SW Portugal. Web Ecol 7:53–62.
Sarmento P, Cruz J, Eira C, Fonseca C (2010) Habitat selection and abundance of common genets Genetta genetta using camera capture-mark-recapture data. Eur J Wildl Res 56:59–66.
Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) (2004) Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan. IUCN/SSC Canid Specialist Group, Gland, Switzerland and Cambridge, UK
Silva JS, Tomé M (2013) Tasmanian blue gum in Portugal – Opportunities and risks of a widely cultivated species. In: Krumm F, Vítková L (eds) Introduced tree species in european forests opportunities and challenges. European Forest Institute, Freiburg, Germany, pp 352–361
Silva-Rodríguez EA, Sieving KE (2012) Domestic dogs shape the landscape-scale distribution of a threatened forest ungulate. Biol Conserv 150:103–110.
Sing T, Sander O, Beerenwinkel N, Lengauer T (2005) ROCR: visualizing classifier performance in R. Bioinformatics 21:3940–3941.
Teixeira D, Carrilho M, Mexia T, Köbel M, Santos MJ, Santos-Reis M, Rosalino LM (2017) Management of Eucalyptus plantations influences small mammal density: evidence from Southern Europe. Ecol Manag 385:25–34.
Teixeira D, Guillera-Arroita G, Hilário RR, Fonseca C, Rosalino LM (2020) Influence of life-history traits on the occurrence of carnivores within exotic Eucalyptus plantations. Divers Distrib 26:1071–1082.
Teixeira D, Ares-Pereira G, Camarinha C, Lima C, Magalhães A, Castro G, Fonseca C, Rosalino LM (2023) Effect of anthropic disturbances on the activity pattern of two generalist mesocarnivores inhabiting Mediterranean forestry plantations. Biodivers Conserv. https://doi.org/10.1007/s10531-023-02548-4
Timo TPC, Lyra-Jorge MC, Gheler-Costa C, Verdade LM (2014) Effect of the plantation age on the use of Eucalyptus stands by medium to large-sized wild mammals in south-eastern Brazil. IForest 8:108–113.
Tomé M, Almeida MH, Barreiro S, Branco MR, Deus E, Pinto G, Silva JS, Soares P, Rodríguez-Soalleiro R (2021) Opportunities and challenges of Eucalyptus plantations in Europe: the Iberian Peninsula experience. Eur J Res 140:489–510.
Torre I, Pulido T, Vilella M, Díaz M (2022) Mesocarnivore distribution along gradients of anthropogenic disturbance in Mediterranean Landscapes. Diversity 14:13. https://doi.org/10.3390/d14020133
Veiras X, Soto MÁ (2011) La conflictividad de las plantaciones de eucalipto en España (y Portugal). Greenpeace, Madrid.
Verdade LM, Rosalino LM, Gheler-Costa C, Pedroso NM, Lyra-Jorge MC (2011) Adaptation of mesocarnivores (Mammalia: Carnivora) to agricultural landscapes in Mediterranean Europe and Southeastern Brazil: a trophic perspective. Middle-sized carnivores in agricultural landscapes. Nova Science Publishers, New York, pp 1–39
Vergara M, Cushman SA, Urra F, Ruiz-González A (2016) Shaken but not stirred: multiscale habitat suitability modeling of sympatric marten species (Martes martes and Martes foina) in the northern Iberian Peninsula. Landsc Ecol 31:1241–1260.
Vilella M, Ferrandiz-Rovira M, Sayol F (2020) Coexistence of predators in time: Effects of season and prey availability on species activity within a Mediterranean carnivore guild. Ecol Evol 10:11408–11422.
Virgós E, Casanovas JG (1997) Habitat selection of genet Genetta genetta in the mountains of central Spain. Acta Theriol (Warsz) 42:169–177
Wickham H (2016) ggplot2: elegant graphics for data analysis, 2nd edn. Springer, New York
Williams ST, Maree N, Taylor P, Belmain SR, Keith M, Swanepoel LH (2018) Predation by small mammalian carnivores in rural agro-ecosystems: an undervalued ecosystem service? Ecosyst Serv 30:362–371.
Zahn A, Rainho A, Rodrigues L, Palmeirim JM (2010) Low macro-arthropod abundance in exotic Eucalyptus Plantations in the Mediterranean. Appl Ecol Environ Res 7:297–301
Zuberogoitia I, Zabala J, Garin I, Aihartza J (2002) Home range size and habitat use of male common genets in the Urdaibai biosphere reserve, Northern Spain. Eur J Wildl Res 48:107–113
Zuur AF, Ieno ENI, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was financially supported by the project POCI-01-0145-FEDER-028204 (WildForests) funded by FEDER, through COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT – Fundação para a Ciência e a Tecnologia, I.P. It was also funded by national funds through FCT, within the scope of the project 2022.03253.PTDC (ForCe). We also thank the University of Aveiro (Department of Biology), and FCT for the financial support to CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020), and to cE3c (UIDB/00329/2020), through national funds and the co-funding by the FEDER within the PT2020 Partnership Agreement and Compete 2020. CHANGE was funded by FCT (LA/P/0121/2020). DFT was supported by a PhD grant (SFRH/BD/131608/2017) from FCT. AJC is supported by a “Juan de la Cierva” contract (IJC2020-042629-I) funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by the European Union Next Generation EU/PRTR. PA is partly funded by LANDINM project (TED2021-132599B-C21) by Ministerio de Ciencia e Innovación/NextGenerationEU.
Author information
Authors and Affiliations
Contributions
The research group is investigating the effects of exotic Eucalyptus plantations’ presence and management on Iberian mammal populations. Author Contributions: DFT, LMR, AJC, and PA, conceived the study; DFT, AJC, DC, and PA formatted and analyzed the data; DFT, LMR, CF, and PA wrote and approved the final manuscript version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teixeira, D.F., Carpio, A.J., Rosalino, L.M. et al. Can Eucalyptus plantations influence the distribution range of mesocarnivores?. Landsc Ecol 38, 3221–3235 (2023). https://doi.org/10.1007/s10980-023-01787-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01787-8