Abstract
Context
Rice, India’s most widely grown crop, suffers substantial and increasing yield loss to insect pests. Insectivorous bats are known suppressors of insect pests, providing significant economic value to agricultural systems worldwide, yet their ecology in Indian agricultural landscapes is poorly understood.
Objectives
We assess the influence of key biotic and abiotic factors on the activity of insectivorous bats over the growing season and within a night in a rice cultivation landscape.
Methods
Passive acoustic recorders were used to track bat activity in a rice field in the Sonitpur district of Assam, India. We used generalised linear mixed models to analyse the effect of temperature, insect activity, and moonlight intensity on the activity of six bat sonotypes. We also used a multimodal analysis to describe the within-night activity patterns of these sonotypes.
Results
Minimum nightly temperature and moonlight intensity had a positive and negative influence, respectively, on the activity of six bat sonotypes, while the activity of four bat sonotypes increased with insect activity. Within-night activity showed one of two patterns: three sonotypes displayed a dusk peak in activity, while the three other sonotypes were active through the night.
Conclusion
The potential to maximise natural pest control in agricultural landscapes can only be realised through understanding the ecology of natural enemies in these landscapes. Our findings suggest that bats in rice fields are tracking insects over a season and within a night, pointing to a valuable ecosystem service in Indian agriculture that is yet to be quantified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is consumed as a staple food by over half the world’s population (IRRI 2019). Varying with geography, climate change is predicted to decrease global rice yields by 3.2 ± 3.7% per degree Celsius increase in mean surface temperature (Zhao et al. 2017). In addition, the increased action of insect pests, which already have severe effects on yield (Pathak and Khan 1994), is predicted to decrease global rice yield by 19% for a 2° Celsius increase in mean temperatures (Deutsch et al. 2018).
Insect pests are hard to control. For many centuries, pest control relied heavily on chemical insecticides (Smith and Secoy 1976), a strategy long recognised as being unsustainable due to the build-up of resistance, biomagnification of chemicals, and a range of health impacts on humans and the ecosystem (Ripper 1956; Baker et al. 2020). One method to reduce our dependence on chemical pesticides is Integrated Pest Management, a cornerstone of which is the use of natural enemies to control pest numbers (Naranjo et al. 2015). Although not widely adopted for a number of reasons, including time and cost effectiveness (Savary et al. 2012; Lou et al. 2013), the control of agricultural pests by augmenting natural enemy populations of parasitoids (Sharma et al. 2019), birds (Long et al. 2013; Karp and Daily 2014; Maas et al. 2016), and fish (Halwart et al. 2012) has been used for many decades. Only in the last decade and a half, however, have insectivorous bats been widely recognised for their role in insect control (Cleveland et al. 2006; Boyles et al. 2011; Maas et al. 2013).
Synchronous cropping, heavy insecticide use and homogenous landscapes that are characteristic of Asian rice fields disproportionately affect the arthropod natural enemies of rice pests (Settle et al. 1996; Bottrell and Schoenly 2012). Unlike most predatory arthropods, many insectivorous bats are opportunistic generalists that can change hunting grounds during the fallow period between seasons, and, during a season, commute long distances from their roost sites to rice fields every night (Marques et al. 2004; Popa-Lisseanu et al. 2009; McCracken et al. 2012). Furthermore, insectivorous bats can consume anywhere from 25% to over 100% of their bodyweight in insects every night (Kunz et al. 2011), and roost in very high densities, some in colonies of over a million individuals (Ghanem and Voigt 2012). Their generalist diet also makes insectivorous bats potential buffers against non-native pest species, against which pre-existing pest control measures may be ineffective. These characteristics make insectivorous bats exceptionally well suited for the control of rice pests. However, it is only by understanding the drivers of bat activity and distribution—spatial, biotic and abiotic—that we can hope, through targeted conservation, to maximise the ecosystem services they perform.
Insectivorous bats have been shown to eat pests of cotton (Federico et al. 2008; Kolkert et al. 2020), corn (Maine and Boyles 2015), rice (Puig-Montserrat et al. 2015; Kemp et al. 2019), cacao (Cassano et al. 2016), coffee (Classen et al. 2014), and a number of other crops (Whitaker et al. 1996). Economic valuations of this service have produced estimates from hundreds of thousands to billions of dollars per year (Cleveland et al. 2006; Boyles et al. 2011; Wanger et al. 2014), to say nothing of the secondary ecological and economic benefits that occur further down-stream (Kunz et al. 2011). Despite the increasing interest in the pest control service of bats, there remains a dearth of studies examining this service in the context of rice (but see Wanger et al. 2014, Puig-Montserrat et al. 2015, Kemp et al. 2019, Sedlock et al. 2019). With a view to maximising the pest control ecosystem service, an economic estimate alone is insufficient unless complemented by a thorough understanding of the biology and ecology of insectivorous bats. This knowledge is sorely lacking in India (Wordley et al. 2015, 2017; Ongole et al. 2018), which boasts 127 species of bats, of which 113 are insectivores (Talmale and Saikia 2018). Our study seeks to address that gap, by describing some of the main temporal drivers of insectivorous bat activity over a rice-dominated landscape in Assam (India), a state which, in 2018, grew rice on 2.43 million hectares of land (Directorate of Economics and Statistics 2019).
Optimal foraging theory postulates that animals employ foraging strategies that maximise net energy gain while minimising risk (Pyke et al. 1977; Lima and Dill 1990). These strategies—a combination of instinctive and learned behaviours—drive decisions behind what to eat, when, where, and for how long. Bats, with their unusual combination of powered flight, high metabolic rates and slow life histories, face a unique set of conditions that influence their foraging behaviour (Barclay and Harder 2003; Welbergen 2006; Prat and Yovel 2020). Powered flight involves high energetic demands and rapid evaporative water loss, significantly so when temperatures are high (Studier 1970). While bats are heterothermic and able to enter torpor to conserve energy, insects, their prey, are ectothermic. Low temperatures significantly decrease the availability of insects, forcing bats either to enter torpor (Yuan et al. 2011; Jonasson and Willis 2012; Stawski et al. 2014) or migrate to warmer climates (Fleming 2010) when their prey is scarce.
Many bats also display an aversion to moonlight, termed ‘lunar phobia’ (Morrison 1978). Lunar phobia is driven by the increased risk of predation from visually hunting nocturnal birds, a risk which increases on brighter nights and disproportionately affects those bats which can be caught by these predators (Lima and O’Keefe 2013; Saldaña-Vázquez and Munguía-Rosas 2013).
Other abiotic factors such as rain (Voigt et al. 2011) and wind speed (Smith and McWilliams 2016) also affect bat activity levels by increasing the energetic costs of flight, but the most important driver of insectivorous bat activity is prey abundance (Egert-Berg et al. 2018; Hałat et al. 2018). Bats go where there is food. For generalist species aiming to maximise energy intake, this means changing not just location but also target prey. To what extent bats use rice fields to hunt is all but unknown, as is their relationship to most rice pests.
Rice in Asia goes through three broad stages of growth: vegetative, reproductive, and maturing or ripening stages (Moldenhauer and Slaton 2001). Arthropods found in rice fields, including pests, follow patterns of population growth tied to their mode of feeding. Detritivores arrive first and peak early (Way and Heong 1994; Sedlock et al. 2019). Herbivores arrive later, to feed on the root, stem, flower, and leaf of the plant, suck sap, or bore into the stem (Heinrichs and Aguda 1994). Different species peak at different points in the season, after which their population declines (Settle et al. 1996). Of the herbivores, the brown planthopper (Nilaparvata lugens) and the white-backed planthopper (Sogatella furcifera) are worth mentioning for being major pests of rice (Catindig et al. 2009) and for being preyed upon by bats (Leelapaibul et al. 2005; Srilophan et al. 2018). Both species peak in population during the late vegetative or reproductive stages of rice growth (Zhong-xian et al. 2006; Sharma et al. 2018).
Given the generalist diets of many insectivorous bats, we hypothesise that pest abundance will be the primary driver of bat activity over rice fields. We further hypothesise that bat activity will increase with increasing temperature and decrease with greater moonlight intensity. Herein, we report the results of a season-long study of bat activity patterns over a rice field in Assam, India.
We identify temporal drivers of bat activity—insect activity, moonlight, and nightly temperature—and infer from these drivers the nature of the relationship between insectivorous bats and atmospheric conditions, and predation pressure, and insect pests of rice over a rice growing season.
We also examine the patterns of bat activity through the night to further illustrate the influence of rice pests on bat behaviour. Studies such as this, in combination with habitat suitability models, exclusion experiments, and economic evaluations, can be used to shape policy to maximise the value of the pest control service provided by bats in the agricultural landscape.
Methods
Study area
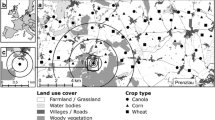
This study was carried out in Puthimari, a village in the Sonitpur district of Assam (26°38′43.0″N, 92°39′55.7″E), and the rice fields adjacent to it. Rice is a major crop in Assam, grown on 2.43 million hectares of land (Directorate of Economics and Statistics 2019). The rice in this village is grown as a mosaic of different strains, with different levels of pesticide and fertiliser use. No fertiliser or inorganic pesticide was added to the crop at the locations of this study. Data on chemical use on neighbouring patches were unavailable. Three rice crops, Ahu, Boro, and Sali, are planted in this village each year in the autumn, summer, and winter, respectively. Our field season was conducted over the Sali/winter rice season. While planting began at the end of July and continued until mid-August, the rice at the six sites used in this study was planted by the 1st of August.
Data Collection
Acoustic data were collected from dusk until between 4:00 am and 4:30 am (beyond which we ran the risk of equipment theft) at the same six locations (Fig. 1) simultaneously over 48 non-consecutive nights between 27 August and 9 December 2019. Six recorders operating simultaneously over the 48 nights collected a total of 2576.5 h of data. Recordings were made using Audiomoth 1.0.0 full spectrum recorders (Hill et al. 2018). The recorders were put into plastic zip-locked bags and placed in shallow (4 cm) baskets attached to bamboo poles, with their microphones pointing upward. The sampling rate was set to the detectors’ maximum of 384 kHz, the gain at medium, and the recorders were programmed to record for ten-min intervals, separated by a one second break.

Source Map of India by Ganeshk (GFDL&cc-by-sa-2.5,2.0,1.0); Airbus, CNES/Airbus, Maxar Technologies, Planet.com
Locations of six Audiomoth recorders in Puthimari village, Assam. Recorders were at least 100 m away from each other, and from the nearest forest. The red section in the map of India marks the state of Assam
The six selected locations were east of the village. Each recorder was placed at a height of 1 m, at least 100 m from its closest neighbour, and also from the nearest forest line. Rice fields extended for over 1 km to the north, south and east of the sites. The impact of artificial light was minimal as the sites were at least 100 m from the nearest house, which were obscured from view by trees. Incidences of shorter recording periods due to logistical or technical issues were accounted for in the analysis. Between 30 September and 1 December 2019, bats were captured in four Avinet mist nets with the help of two Peersonic bat lures. These bats were weighed and photographed and they were recorded with a Pettersson D240X recorder set to ‘Time Expansion’ mode upon release. Permission for this was granted by the Office of the Divisional Forest Officer, Sonitput West Division, Tezpur. Animal ethics approval was granted by the Institutional Animal Ethics Committee (IAEC) of the National Centre for Biological Sciences. The IAEC approval number is NCBS-IAE-2018/01(ME).
Acoustic data analysis
The raw acoustic data were processed using custom written code in Python version 2.7 (Rossum et al. 1995) to isolate bat calls and extract key measurements of the call, as follows. (1) Frequency of maximum energy (FMAXE): the frequency containing the most energy in the call (Wordley et al. 2014). (2) Minimum and maximum frequencies: calculated as the lowest and highest frequencies that contained 5% of FMAXE. (3) Bandwidth: the difference between the minimum and maximum frequency. (4) Call length: the time between when the call first crosses 5% FMAXE, and when it last crosses 30% FMAXE. (5) Average amplitude: the average amplitude of the call. Once extracted, calls were classified into sonotypes using a MatLab Classification learner (The MathWorks Inc. 2019). Training data for each sonotype were created by extracting the parameters of manually identified calls. These training data were used by the classification learner in a linear discriminant analysis to classify all the extracted calls into sonotypes. Following this, the classified calls were run through a final round of processing in R version 3.63 (R Core Team 2020), where hard limits on FMAXE and bandwidth were used to reclassify calls that had been misclassified into a similar sonotype. The calls were then filtered to exclude undesirable sections of the audio, such as during heavy rain, instrumental failure resulting in static, or bats recorded prior to the recorders being in position.
Due to the absence of a complete acoustic library for Indian insectivorous bats, calls were classified to the sonotype level instead of species. Sonotypes were defined based on FMAXE and bandwidth into the following classes: (1) Constant Frequency calls (CF), and (2) Frequency modulated-Quasi Constant frequency calls (FM-QCF). One pure QCF sonotype was detected but could not be distinguished from a similar FM-QCF call. The two sonotypes were merged into one FM-QCF sonotype. Pure FM, and FM-CF-FM calls were not recorded. Constant frequency calls, although detected in the data, were not included in the analysis due to the inability of the classifier to separate them from ultrasonic insect noises, which occurred at the same frequency. Taken forward for analysis were six FM-QCF sonotypes, which were identified by FMAXE values at 20 kHz, 31 kHz, 34 kHz, 38 kHz, 48 kHz, and 65 kHz, respectively.
The classification of calls was performed using the classification learner app in MATLAB (The MathWorks Inc. 2019), using a template created by manually identifying calls and extracting relevant parameters. Every recording was divided into five second intervals and a bat pass was defined as any interval with more than two calls of the same sonotype (Millon et al. 2015). We undertook a manual check of 10% of the ~ 20,000 extracted passes, selected by a random number generator, to verify the accuracy of the classification. The classifier accurately identified over 90% of all passes. Code for the classifier can be found at https://github.com/IqbalBhalla/BatClassifier.git.
Statistical analysis
All statistical analysis was conducted in R. We used generalised linear mixed model (GLMM) to analyse the relationship between bat activity and its drivers (McCullagh and Nedler 2019). A total of 267 sampling units were used in each statistical model (6 recorders over 48 nights, minus nights where the recorders failed) Prior to building the model, outliers in the response variable ‘bat activity’ were identified and deleted using the ’check outliers’ function from the ’Performance’ R package (Lüdecke et al. 2020). All the variables were then standardised to zero mean and unit variance before analysis so that their effect sizes were comparable (Schielzeth 2010).
The following variables were considered relevant drivers of bat activity. (1) Temperature: minimum nightly temperature was included to test for the effect of temperature on bat activity. For those sonotypes which showed a quadratic relationship between activity and minimum nightly temperature, minimum nightly temperature squared was included as a variable in their supermodel. (2) Moonlight: the percentage of the moon visible on each night, was included to test for the effect of moonlight intensity on bat activity. Cloud cover would impact moonlight intensity, but as we were unable to quantify this at a relevant resolution, we included percentage of the moon visible as a measure that strongly correlated with brightness. Temperature and moonlight data for Puthimari village were obtained, with permission, from the World Weather Online database (WorldWeatherOnline.com). (3) Sampling effort: the recorders failed unexpectedly throughout the field season, leaving gaps in the dataset ranging from one to ten hours. To account for this, and for the logistical challenges that prevented the recording from starting on time on some nights, the total number of minutes sampled on any night was included as the factor ‘sampling effort’. (4) Ambient insect noise: since the recorders were placed in the middle of rice fields, ambient noise above 5 kHz was made up primarily of insects (frogs dominated frequencies below 5 kHz). Natural and anthropogenic noise, such as bird calls and firecrackers, were occasional, and vastly outweighed by the continuous insect noise. This measure was therefore included as a proxy for the activity of vocal insects. The ambient noise of each night was calculated by taking the mean amplitude between 5 and 24 kHz, aggregated from all six recorders, and divided by the sampling effort of that particular night. (5) Day of the season: the number of days from 1st August 2019, which was the date by which rice at all six sites had been planted. This variable reflects the linear effect of rice maturity on bat activity, and seasonal changes in bat activity. Several major pests of rice—including leafhoppers and planthoppers—which are known prey of bats, are not vocal. Because these pests are known to peak in the middle growth stages of the rice (Heong et al. 1992; Zhong-xian et al. 2006), we also included in the model the quadratic transformation of the ‘Day of the season’ variable—‘Day of the season squared’—to capture a possible activity pattern that was high in the middle of the season and low at either end.
Two-way interactions between predictors were included in our analysis on an a priori basis when there was a biological reason to do so (Harrison et al. 2018). These were interactions between: (1) moonlight and minimum temperature, (2) moonlight and ambient insect noise, and (3) minimum temperature and ambient insect noise. Also included in the global model was the random effect of ‘site’—the six locations that the recorders were placed in—to account for non-independence of datapoints collected from the same location over multiple days.
Prior to running the model, all explanatory variables were tested for collinearity using Spearman’s rank correlation, with a threshold of |0.7| to determine whether one factor had to be dropped (Dormann et al. 2013). Day of the season was highly correlated with minimum nightly temperature (Spearman’s rank correlation: r = − 0.79), while day of the season squared was highly correlated with ambient insect noise (Spearman’s rank correlation: r = − 0.90). Therefore, both day of the season and its quadratic—‘day of the season squared’—as well as all relevant interactions were removed from further analysis.
The remaining variables and interactions were included in a global model implemented with the ‘glmmTMB’ package (Brooks et al. 2017). The model was initially tested with a Poisson error distribution, since the response variable took the form of count data, but this was changed to a quasi-Poisson when the model tested positive for overdispersion (Bolker et al. 2009).
An information theoretic approach was then implemented to capture the best set of variables explaining bat activity by fitting all possible models and calculating Akaike’s information criterion corrected for sample size (AICc) for each model; the best model being the one with the smallest AICc (Burnham and Anderson 2002). This was done using the ‘dredge’ function from the ‘MuMIn’ package (Bartoń 2022), which compared all possible combinations of variables in the global model. Note that during the selection procedure, an interaction was included in a model only if its composite variables were also included, even if the variables independently were not statistically significant. All models with a ΔAICc value < 2 (the difference between each model’s AICc and the lowest AICc) were considered as receiving equal statistical support (Burnham and Anderson 2002). When the ‘dredge’ function returned more than one model having an AICc within two units of the best model, the model with fewest variables was chosen (Symonds and Moussalli 2011). The top ten models of each sonotype can be found in Supplementary Material Table S.I–S.VII.
One ‘best model’ was identified for each sonotype, as well as one model for all the sonotypes together. All best models were tested for overdispersion and zero inflation using the ‘testZeroInflation’ and ‘testDispersion’ functions from the DHARMa package (Hartig 2022). The residuals were analysed using the ‘simulateResiduals’ function, also from the DHARMa package. Residuals were checked for obvious patterns (heteroscedasticity, linear/quadratic trends), as well as deviance in the Kolgomorov–Smirnov test. A pseudo R-squared value based on the likelihood ratio test was calculated using the function ‘r.squaredLR’ from the ‘MuMIn’ package. Finally, for a given model, the absolute values of the standardised regression coefficients were rescaled to sum to one to derive a measure of relative importance for each predictor.
Temporal distribution of nightly activity
Bat activity was also analysed for patterns over the night. Each sonotype was analysed separately, with calls being aggregated into ten-min intervals starting from dusk.
First, a clustering algorithm based on a parameterised finite Gaussian mixture model was fitted to the data to identify clusters within the nightly activity data using the package and function ’Mclust’ in R (Scrucca et al. 2016). Second, the mean and sigma of each cluster recovered from the previous analyses were then used in multimodal analyses to fit multiple normal distributions that best describe the nightly activity pattern of each sonotype. This was implemented with the ‘mix’ function from the ‘MixDist’ package (Macdonald and Pitcher 1979; Macdonald and Du 2018). To standardise for changing dusk times, data from each night were clipped to begin 20 min post-dusk and end 620 min post-dusk. This technique was developed originally to analyse the size frequency distribution of a population with overlapping age groups (Kell and Kell 2011). This, to the best of our knowledge, is the first time that this approach has been implemented to study temporal patterns in nightly bat activity.
Results
We identified a total of 18,890 passes from six sonotypes over a period of 48 nights of recording. All six sonotypes were FM-QCF and featured FMAXE values around 20 kHz, 31 kHz, 34 kHz, 38 kHz, 48 kHz, and 65 kHz, respectively. Henceforth these sonotypes will be referred to using ‘S’ followed by their identifying frequency (e.g. S20).
The most common sonotype was S38, accounting for 40.1% percent of all passes, followed by S48 (21.1%), S34 (15.3%), S65 (9.1%), S31 (8.9%), and S20 (5.3%). Nightly activity of all sonotypes peaked between 25 September and 28 October—which corresponded to the late vegetative/reproductive stages of rice growth—with all but S34 peaking between the 15th and 28th of October.
Variables affecting bat activity
Overall, our models showed low to moderate R-squared values (0.166–0.389), which was to be expected given the many interacting factors that influence bat activity. Total bat activity over rice fields (best supported model R2 = 0.262, Supplementary Material Table S.VIII) increased with sampling effort (estimate = 0.291), ambient insect noise (0.237), minimum nightly temperature (0.164) and the interaction between ambient insect noise and moonlight intensity (0.100), but decreased with the interaction between ambient insect noise and minimum temperature (− 0.213) and moonlight intensity (− 0.126; Table S.VIII).
The activity of all six sonotypes increased with sampling effort, indicating that more bats were recorded when the number of hours sampled in a night was higher. The activity of three sonotypes (S31, S34, and S48) increased with ambient insect noise, a proxy for vocal insect activity. For sonotype S38, ambient insect noise was significant only as part of an interaction with minimum nightly temperature. Minimum nightly temperature was a significant and positive driver of the activity of all sonotypes, apart from S48, for which minimum nightly temperature was only significant as part of the interaction with ambient insect noise (Fig. 2). The activity of S31 decreased with minimum nightly temperature squared, indicating that activity was lower during both very high and very low temperatures. Finally, the activity of every sonotype, apart from S31, decreased with moonlight intensity (Fig. 2).
The standardized estimate (circle) and 95% confidence interval (wings) of all factors (standardised) and interactions in the best model for each sonotype. Generalised linear mixed models for each sonotype were built on data from 48 nights of sampling at six locations in a rice field in Puthimari, Assam. ‘Best’ models were identified by comparing subsets of a global model using AICc. Solid circles indicate p-value < 0.05, hollow circles indicate p-value > 0.05. ‘x’ symbols indicate variables included in the global models that were not present in the ‘best’ models of each sonotype
Minimum nightly temperature squared had a significant and negative effect in the best model of S31, indicating that activity was lower during both very high and very low temperatures. Moonlight intensity was a significant and a negative driver of the activity of five sonotypes (S20, S34, S38, S48, and S65).
The effect of the interaction between ambient insect noise and minimum nightly temperature was significant and negative in the models of four out of six sonotypes. On cold nights—the left end of Fig. 3b–e—bat activity was significantly higher when ambient insect noise was higher. On warm nights (the right end of Fig. 3b–e), when the energetic costs of flight are lower, activity increased when insects were scarce.
The effects of two-way interactions on bat activity. Generalised linear mixed models for each sonotype were built on data from 48 nights of sampling at six locations in a rice field in Puthimari, Assam. Plots a to f show how the interactions between fixed effects in the model (minimum nightly temperature, moonlight intensity, and ambient insect noise) influence bat activity in those models where interactions are significant. All three variables are standardized, as they are in the model
The interaction of ambient insect noise and moonlight intensity was significant and positive in the model for S48. High levels of insect activity reduced the effect of bright nights on the activity of S48 (Fig. 3f). The interaction between moonlight and minimum nightly temperature was significant and positive for one sonotype. The activity of S20 was lower with the combination of low temperatures and high moonlight than it was with less extreme values of either variable (Fig. 3a).
Temporal distribution of nightly activity
The multimodal analysis of nightly activity patterns of each sonotype produced two broad patterns. The first, exhibited by S31, S34 and S38 (Fig. 4b–d), show a primary peak early in the night, at 32, 38, and 88 min past dusk, respectively, followed by one to three smaller peaks through the night. The second pattern, seen in S20, S48 and S65 (Fig. 4a, e, and f), exhibited a more even distribution through the night, with the second or third peak being the highest. Details of the locations and standard error of each peak, as well as results of the chi-square goodness of fit test can be found in Supplementary Material (Table S.IX).
The temporal distribution of nightly activity of six sonotypes. Data were collected at six locations over 48 nights of sampling in a rice field in Puthimari, Assam. Every night was divided into ten-min intervals starting at dusk and ending ten hours post dusk (blue bars). The number of passes of each sonotype in each ten-min post-dusk interval was summed up over all 48 nights of sampling. Nightly activity was clipped from 20 min post dusk until 620 min post dusk. Composite normal distributions that best fit the overall pattern of nightly activity of each sonotype (red lines) were fit using the ‘mix’ function, verified using a Chi-square goodness of fit test. S20 has an additional peak caused by the outlier at 190 min that is not visible due to a small standard deviation
Discussion
We describe the activity of insectivorous bats foraging over rice fields, both over a rice season, and over the course of the night. In addition, we identify how biotic and abiotic drivers influence bat activity over the rice season.
Activity over the season
Few studies have considered bats in rice-dominated landscapes. Most that do so focus on bat diet and have established clear links between insectivorous bats and rice pests (Leelapaibul et al. 2005; Puig-Montserrat et al. 2020). Kemp et al. (2019) showed that insectivorous bats in Madagascar hunted over rice fields preferentially compared to nearby forested areas. Similarly, Puig-Montserrat et al. (2015) found that insectivorous bats in Iberian rice fields significantly increase their activity when moth activity increases. The moth in question was the rice stem borer (Chilo supressalis), one of the most significant rice pests in Asia (Yao et al. 2017). Puig-Montserrat et al. (2015) went further and installed bat boxes to increase the resident populations of insectivorous bats. After ten years, pest control by bats was so effective that the need for pesticides was eliminated. The direct financial value placed on this service was 21 Euros per hectare per annum. It is only the second valuation of the bat pest control service on rice, after Wanger et al. (2014) estimated this service to be worth 1.2 million USD per annum in Thailand. Our results indicate that three out of six sonotypes, which account for 67.4% of insectivorous bat activity in the study area, were positively influenced by our proxy of insect activity over the rice season. We started the analysis intending to use two proxies for insect activity: ambient insect noise and the quadratic function of day-of-the-season. The latter represented population growth patterns of important rice pests that were documented prey of bats (Zhong-xian et al. 2006; Sharma et al. 2018). We were forced to drop this term due to the high covariation with ambient insect noise. The same covariation, however, implied one of two things. Either the two variables were acting as a proxy for the same insect community, or since many of the major rice pests are not vocal, alternatively the vocal insects, which may be pests themselves, have similar population growth patterns to non-vocal rice pests such as planthoppers and leafhoppers. It is ecologically parsimonious to assume that they grow more abundant as the rice matures, peaking in the middle stages of rice growth, after which their population declines (Heong et al. 1992; Zhong-xian et al. 2006; Sharma et al. 2018). The mid-season peak, documented in pests and now seen in vocal insects, was tracked by bats belonging to the sonotypes S31, S34, and S48. The strength of this effect suggests that the bats recorded at our sites were responding to changes in rice pest abundance by preferentially hunting over rice fields when insect activity was high.
While insect activity was a major driver of bat activity, it was not the only one. Although temporal heterothermy in Indian bats has not been studied, data from other tropical and subtropical bats suggest that insectivorous bats are likely to be heterothermic and capable of entering torpor to reduce energy expenditure on cold nights (Stawski et al. 2014). Temperatures at our field site fell to a minimum of 11 °C. Studies have shown that bats can have normothermic ranges above 20 °C, below which metabolic rates and resting activity patterns are affected (Soriano et al. 2002; Currie et al. 2014). While no such studies have focussed on Indian bats, it is reasonable to expect that bats in our study site, where summer temperatures regularly exceed 30 °C have a high normothermic range, and in temperatures approaching 11 °C would exhibit sub-normothermic behaviour (Ramos Pereira et al. 2022). In addition, such low temperatures also affect insect activity (Stejskal et al. 2019), which in turn would influence bat activity.
Whether the effect is direct or indirect, given that this pattern has been extensively documented before (Scanlon and Petit 2008; Barros et al. 2014; Wolbert et al. 2014; Appel et al. 2019), we predicted that bat activity would be positively correlated with temperature. This was true for five out of six sonotypes. The sixth sonotype, S31, was influenced by minimum nightly temperature squared, indicating that its activity is reduced at both low and high temperatures, presumably on either side of its normothermic range.
The field site hosted an abundance of owls (Iqbal Bhalla, personal observation). We hypothesised that these nocturnal avian predators—would impose predation pressure on slower bat species that they could hunt, and that the risk of predation would be greater on brighter nights. This is especially true in the open landscape of a rice field, where bats are more visible against the monotonous backdrop and have less cover due to a lack of trees. Similar to previous studies (Morrison 1978; Meyer et al. 2004; Lima and O’Keefe 2013; Appel et al. 2017), we found that five out of six sonotypes decrease their activity on brighter nights. While factors such as temperature, moonlight, and insect activity individually affect each of these considerations, our analysis suggests that combinations of them can shift the cost-risk-benefit balance. This has been suggested before (Appel et al. 2019), but rarely investigated. The interaction terms of our models explored these combinations.
To maximise fitness, a bat must employ a foraging strategy that efficiently weighs the cost-benefit trade-off between the risk of predation and the reward of successful hunting. Bright moonlight imposes a significant predation risk on bats, reducing their activity. However, as seen in Fig. 3f, bright nights suppress the activity of S48 to a lesser degree when there is greater insect activity, implying that this sonotype is more likely to risk high levels of predation when the nutritional gains or prey detectability are higher. This, in part, concurs with recent studies which described bats increasing their activity, or moving to open spaces, on moonlit nights to maximise hunting success (Roeleke et al. 2018; Kolkert et al. 2020). A similar pattern is seen for sonotype S20, where the activity is lower with increasing moonlight, but this effect is less pronounced when temperatures are high. The activity of each of S31, S34, S38, and S48 was lower when temperatures and insect activity were low, pointing to an additive effect prompting reduced bat activity when insects are both less vocal and less active.
Nightly activity patterns
The activity patterns of the six sonotypes through the night are consistent with two primary foraging modes. The dusk peaks seen in three sonotypes (S31, S34, and S38) are characteristic of bats hunting crepuscular insects that also show dusk peaks in activity (Rydell et al. 1996; O’Donnell 2000). While these insects may peak in activity earlier than bats, predation pressure from diurnal raptors delays the emergence of many bats, which then have a short window to maximise their feeding (Speakman 1991). Both planthoppers and leafhoppers, as well as other rice pests, which show a peak in population size during the middle of the rice growing season, exhibit daily peaks in activity around dusk (MacQuillan 1975; Kersting and Başpmar 1995; Zhu et al. 2017; Mazza et al. 2020). Both patterns of activity—over a season and within a night—were also seen in two sonotypes, S31 and S34, lending support to the argument that they are tracking these insect pests.
The second broad pattern in nightly activity points to bats that hunt nocturnal prey that are active, or can be caught by gleaning, for a significant proportion of the night (Jones and Rydell 1994). Without the incentive to emerge early and risk predation by diurnal predators, these bats emerge later and have a less skewed activity pattern over the night. Although nocturnal predators still impose predation pressure, this risk is more evenly distributed through the night. As a result, the three sonotypes which exhibited this pattern, S20, S48, and S65, had a more even nightly activity pattern, with only S20 exhibiting a clear peak around midnight. However, it is important to note that we did not have confirmed bat species identification on our sonotypes, or information on roost location, and therefore cannot be certain about the impact of distance from roosts on the observed patterns of activity.
Conclusion
Bats in India are under-studied. Despite evidence from around the world of their importance as bioindicators (Jones et al. 2009) and providers of ecosystem services (Kunz et al. 2011), only a handful of studies have investigated the ecology of insectivorous bats in India (Wordley et al. 2015, 2017; Ongole et al. 2018). Any attempt to maximise the ecosystem services of insectivorous bats can only be made through an understanding of the requirements, limitations, and drivers of their distribution and activity. As shown in Fig. 1, the six recorders used in this study were placed in a rice field adjacent to a village, with each recorder being at least 100 and no more than 150 m from its nearest neighbour. Moving further away from the village, would create a gradient of increasing distance to the nearest treeline, and all the associated microhabitat conditions created by a patch of semi-forested area. This would undoubtedly influence insect activity between the recorders, and in turn bat activity, both as a consequence of the above factors and because of the insect activity itself. While the current study did not assess microclimatic conditions or record insects to the species level, future studies should examine the influence of the surrounding landscape on insect activity, particularly the effect of distance to the nearest treeline, in parallel to bat activity. With species or genera level information on insect activity, one can infer the relationship between specific pests and the bats that might hunt them, which would be extremely valuable to furthering our understanding of pest control by bats.
This study contributes to our understanding of the role of bats in pest control by demonstrating how insectivorous bats hunting in rice-dominated landscapes are influenced by insect activity, temperature and moonlight. Our findings that bats and insects have similar activity patterns in agricultural fields, not just over a season but also over the night, suggest that bats hunt insect populations over rice fields, and are therefore likely to provide pest control ecosystem services. The contribution of bats to pest control in rice fields is supported by the findings of an exclusion experiment conducted in the same fields, which showed that rice health, but not total yield, is negatively affected by the exclusion of bats (Bhalla et al. 2023).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Appel G, López-Baucells A, Ernest-Magnusson W, Bobrowiec P (2017) Aerial insectivorous bat activity in relation to moonlight intensity. Mammalian Biology 85:37–46.
Appel G, Lopez-Baucells A, Magnusson W, Bobrowiec P (2019) Temperature, rainfall, and moonlight intensity effects on activity of tropical insectivorous bats. J Mammal 100:1–12
Baker BP, Green TA, Loker AJ (2020) Biological control and integrated pest management in organic and conventional systems. Biol Control 140:104095
Barclay RM, Harder LD (2003) Life histories of bats: life in the slow lane. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, p 253
Barros MAS, Pessoa DMA, Rui AM (2014) Habitat use and seasonal activity of insectivorous bats (Mammalia: Chiroptera) in the grasslands of southern Brazil. Zoologia (Curitiba) 31:153–161
Bartoń K (2022) MuMIn: Multi-Model Inference. R package version 1.46.0. https://cran.r-project.org/package=MuMIn. Accessed 2022
Bhalla IS, Aguirre-Gutiérrez J, Whittaker RJ (2023) Batting for rice: the effect of bat exclusion on rice in North-East India. Agric Ecosyst Environ 341:108196
Bolker BM, Brooks ME, Clark CJ et al (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bottrell DG, Schoenly KG (2012) Resurrecting the ghost of green revolutions past: the brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J Asia Pac Entomol 15:122–140
Boyles JG, Cryan PM, McCracken GF, Kunz TH (2011) Economic importance of bats in agriculture. Science 332:41–42
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cassano CR, Silva RM, Mariano-Neto E et al (2016) Bat and bird exclusion but not shade cover influence arthropod abundance and cocoa leaf consumption in agroforestry landscape in northeast Brazil. Agric Ecosyst Environ 232:247–253
Catindig JLA, Arida GS, Baehaki SE et al (2009) Situation of planthoppers in Asia. Planthoppers: new threats to the sustainability of intensive rice production systems in Asia. International Rice Research Institute Los Baños, Philippines, pp 191–220
Classen A, Peters MK, Ferger SW et al (2014) Complementary ecosystem services provided by pest predators and pollinators increase quantity and quality of coffee yields. Proc Royal Soc B: Biol Sci 281:20133148
Cleveland CJ, Betke M, Federico P et al (2006) Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front Ecol Environ 4:238–243
Currie SE, Körtner G, Geiser F (2014) Heart rate as a predictor of metabolic rate in heterothermic bats. J Exp Biol 217:1519–1524
Deutsch CA, Tewksbury JJ, Tigchelaar M et al (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919
Directorate of Economics and Statistics (2019) Agricultural statistics at a glance 2019. Ministry of Agriculture, Government of India
Dormann CF, Elith J, Bacher S et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Egert-Berg K, Hurme ER, Greif S et al (2018) Resource ephemerality drives social foraging in bats. Curr Biol 28:3667–3673
Federico P, Hallam TG, McCracken GF et al (2008) Brazilian free-tailed bats as insect pest regulators in transgenic and conventional cotton crops. Ecol Appl 18:826–837
Fleming TH (2010) Bat migration. In: Breed MD, Moore J (eds) Encyclopedia of animal behavior, 2nd edn. Oxford Academic Press, Oxford, pp 145–149
Ghanem SJ, Voigt CC (2012) Increasing awareness of ecosystem services provided by bats. Adv Study Behav 44:279–302
Hałat Z, Dechmann DKN, Zegarek M et al (2018) Sociality and insect abundance affect duration of nocturnal activity of male parti-colored bats. J Mammal 99:1503–1509
Halwart M, Litsinger JA, Barrion AT et al (2012) Efficacy of common carp and Nile tilapia as biocontrol agents of rice insect pests in the Philippines. Int J Pest Manage 58:330–346
Harrison XA, Donaldson L, Correa-Cano ME et al (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794–e4794
Hartig F (2022) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.5. http://florianhartig.github.io/DHARMa/. Accessed 2022
Heinrichs EA, Aguda RM (1994) Biology and management of rice insects. Wiley, New York
Heong KL, Aquino GB, Barrion AT (1992) Population dynamics of plant- and leafhoppers and their natural enemies in rice ecosystems in the Philippines. Crop Prot 11:371–379
Hill AP, Prince P, Piña Covarrubias E et al (2018) AudioMoth: evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods Ecol Evol 9:1199–1211
IRRI (2019) World Rice Statistics. International Rice Research Institute
Jonasson KA, Willis CKR (2012) Hibernation energetics of free-ranging little brown bats. J Exp Biol 215:2141–2149
Jones G, Rydell J (1994) Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Philos Trans: Biol Sci 346:445–455
Jones G, Jacobs DS, Kunz TH et al (2009) Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8:93–115
Karp DS, Daily GC (2014) Cascading effects of insectivorous birds and bats in tropical coffee plantations. Ecology 95:1065–1074
Kell LT, Kell A (2011) A comparison of age slicing and statistical age estimation for Mediterranean Swordfish (Xiphias gladius). Collect Vol Sci Pap ICCAT 66:1522–1534
Kemp J, López-Baucells A, Rocha R et al (2019) Bats as potential suppressors of multiple agricultural pests: a case study from Madagascar. Agric Ecosyst Environ 269:88–96
Kersting U, Başpmar H (1995) Seasonal and diurnal flight activity of Circulifer haematoceps (Hom., Cicadellidae), an important leafhopper vector in the Mediterranean area and the Near East. J Appl Entomol 119:533–537
Kolkert H, Andrew R, Smith R et al (2020) Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol Evol 10:371–388
Kunz TH, Braun de Torrez E, Bauer D et al (2011) Ecosystem services provided by bats. Ann N Y Acad Sci 1223:1–38
Leelapaibul W, Bumrungsri S, Pattanawiboon A (2005) Diet of wrinkle-lipped free-tailed bat (Tadarida plicata Buchannan, 1800) in central Thailand: insectivorous bats potentially act as biological pest control agents. Acta Chiropterologica 7:111–119
Lima S, Dill L (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zoology- 68:619–640
Lima SL, O’Keefe JM (2013) Do predators influence the behaviour of bats? Biol Rev 88:626–644
Long P, Huang H, Liao X et al (2013) Mechanism and capacities of reducing ecological cost through rice-duck cultivation. J Sci Food Agric 93:2881–2891
Lou Y-G, Zhang G-R, Zhang W-Q et al (2013) Biological control of rice insect pests in China. Biol Control 67:8–20
Lüdecke D, Makowski D, Waggoner P, Patil I (2020) performance: assessment of regression models performance. CRAN. https://doi.org/10.5281/zenodo.3952174
Maas B, Clough Y, Tscharntke T (2013) Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol Lett 16:1480–1487
Maas B, Karp DS, Bumrungsri S et al (2016) Bird and bat predation services in tropical forests and agroforestry landscapes. Biol Rev 91:1081–1101
Macdonald P, Du J (2018) mixdist: Finite Mixture Distribution Models. R package version 0.5-5. https://ms.mcmaster.ca/peter/mix/mix.html. Accessed 2018
Macdonald PDM, Pitcher TJ (1979) Age-Groups from size-frequency data: a versatile and efficient method of analyzing distribution mixtures. J Fish Res Board Can 36:987–1001
MacQuillan MJ (1975) Seasonal and diurnal flight activity of Nilapavata lugens STAL (Hemiptera: Delphacidae) on Guadalcanal. Appl Entomol Zool 10:185–188
Maine JJ, Boyles JG (2015) Bats initiate vital agroecological interactions in corn. Proc Natl Acad Sci USA 112:12438–12443
Marques JT, Rainho A, Carapuço M et al (2004) Foraging behaviour and habitat use by the european free-tailed bat Tadarida teniotis. Acta Chiropterologica 6:99–110
Mazza G, Marraccini D, Mori E et al (2020) Assessment of color response and activity rhythms of the invasive black planthopper Ricania speculum (Walker, 1851) using sticky traps. Bull Entomol Res 110:480–486
McCracken GF, Westbrook JK, Brown VA et al (2012) Bats track and exploit changes in insect pest populations. PLoS ONE 7:e43839–e43839
McCullagh P, Nedler JA (2019) Generalized linear models. Routledge, Abingdon
Meyer CFJ, Schwarz CJ, Fahr J (2004) Activity patterns and habitat preferences of insectivorous bats in a west african forest-savanna mosaic. J Trop Ecol 20:397–407
Millon L, Julien J-F, Julliard R, Kerbiriou C (2015) Bat activity in intensively farmed landscapes with wind turbines and offset measures. Ecol Eng 75:250–257
Moldenhauer K, Slaton N (2001) Rice growth and development. Rice Prod Handb 192:7–14
Morrison DW (1978) Foraging ecology and energetics of the frugivorous bat Artibeus jamaicensis. Ecology 59:716–723
Naranjo SE, Ellsworth PC, Frisvold GB (2015) Economic value of biological control in integrated pest management of managed plant systems. Ann Rev Entomol 60:621–645
O’Donnell C (2000) Influence of season, habitat, temperature, and invertebrate availability on nocturnal activity of the New Zealand long-tailed bat (Chalinolobus tuberculatus). New Z J Zool 27:207–221
Ongole S, Sankaran M, Karanth KK (2018) Responses of aerial insectivorous bats to local and landscape-level features of coffee agroforestry systems in western ghats, India. PLoS ONE 13:e0201648
Pathak MD, Khan ZR (1994) Insect pests of rice. International Rice Research Institute, Manila, Philippines
Popa-Lisseanu A, Bontadina F, Ibáñez C (2009) Giant noctule bats face conflicting constraints between roosting and foraging in a fragmented and heterogeneous landscape. J Zool 278:126–133
Prat Y, Yovel Y (2020) Decision making in foraging bats. Curr Opin Neurobiol 60:169–175
Puig-Montserrat X, Torre I, López-Baucells A et al (2015) Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mamm Biol 80:237–245
Puig-Montserrat X, Flaquer C, Gómez-Aguilera N et al (2020) Bats actively prey on mosquitoes and other deleterious insects in rice paddies: potential impact on human health and agriculture. Pest Manag Sci 76:3759–3769
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Ramos Pereira MJ, Stefanski Chaves T, Bobrowiec PE, Selbach Hofmann G (2022) How aerial insectivore bats of different sizes respond to nightly temperature shifts. Int J Biometeorol 66:601–612
R Core Team (2020) R: A language and environment for statistical computing
Ripper WE (1956) Effect of pesticides on balance of arthropod populations. Ann Rev Entomol 1:403–438
Roeleke M, Teige T, Hoffmeister U et al (2018) Aerial-hawking bats adjust their use of space to the lunar cycle. Mov Ecol 6:11
Rossum VG Jr, Drake LF (1995) Python reference manual. Centrum voor Wiskunde en Informatica, Amsterdam
Rydell J, Entwistle A, Racey PA (1996) Timing of foraging flights of three species of bats in relation to insect activity and predation risk. Oikos 76:243–252
Saldaña-Vázquez RA, Munguía-Rosas MA (2013) Lunar phobia in bats and its ecological correlates: a meta-analysis. Mammalian Biology 78:216–219
Savary S, Horgan F, Willocquet L, Heong KL (2012) A review of principles for sustainable pest management in rice. Crop Prot 32:54–63
Scanlon AT, Petit S (2008) Effects of site, time, weather and light on urban bat activity and richness: considerations for survey effort. Wildl Res 35:821–834
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Scrucca L, Fop M, Murphy TB, Raftery AE (2016) Mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R J 8:289–317
Sedlock J, Stuart A, Horgan F et al (2019) Local-scale bat guild activity differs with rice growth stage at ground level in the Philippines. Diversity 11:148
Settle W, Ariawan H, Astuti E et al (1996) Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 77:1975–1988
Sharma K, Raju S, Jaiswal D (2018) Influence of environmental effect on the population dynamics of brown plant hopper, Nilaparvata lugens (Stal) and white–backed plant hopper, Sogatella furcifera (Hovarth) in Varanasi region. J Entomol Res 42:339
Sharma S, Shera P, Sangha K (2019) Species composition of parasitoids and predators in two rice agro-farming systems-effect of ecological intensification. Int J Trop Insect Sci 40:233–238
Smith AD, McWilliams SR (2016) Bat activity during autumn relates to atmospheric conditions: implications for coastal wind energy development. J Mammal 97:1565–1577
Smith AE, Secoy DM (1976) A compendium of inorganic substances used in european pest control before 1850. J Agric Food Chem 24:1180–1186
Soriano PJ, Ruiz A, Arends A (2002) Physiological responses to ambient temperature manipulation by three species of bats from andean cloud forests. J Mammal 83:445–457
Speakman JR (1991) The impact of predation by birds on bat populations in the British Isles. Mammal Rev 21:123–142
Srilophan S, Bumrungsri S, Jantarit S (2018) The wrinkle-lipped free-tailed bat (Chaerephon plicatus Buchannan, 1800) feeds mainly on brown planthoppers in rice fields of central Thailand. Acta Chiropterologica 20:207–219
Stawski C, Willis CKR, Geiser F (2014) The importance of temporal heterothermy in bats. J Zool 292:86–100
Stejskal V, Vendl T, Li Z, Aulicky R (2019) Minimal thermal requirements for development and activity of stored product and food industry pests (Acari, Coleoptera, Lepidoptera, Psocoptera, Diptera and Blattodea): a review. Insects 10:149
Studier EH (1970) Evaporative water loss in bats. Comp Biochem Physiol 35:935–943
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Talmale S, Saikia U (2018) A checklist of indian bat species (Mammalia: Chiroptera) version 2.0. Zoological Survey of India
The MathWorks Inc (2019) MATLAB and Statistics Toolbox Release 2019b. Natick, Massachusetts
Voigt CC, Schneeberger K, Voigt-Heucke SL, Lewanzik D (2011) Rain increases the energy cost of bat flight. Biol Lett 7:793–795
Wanger T, Darras K, Bumrungsri S et al (2014) Bat pest control contributes to food security in Thailand. Biol Conserv 171:220–223.
Way MJ, Heong KL (1994) The role of biodiversity in the dynamics and management of insect pests of tropical irrigated rice—a review. Bull Entomol Res 84:567–587
Welbergen JA (2006) Timing of the evening emergence from day roosts of the grey-headed flying fox, Pteropus poliocephalus: the effects of predation risk, foraging needs, and social context. Behav Ecol Sociobiol 60:311
Whitaker JO, Neefus C, Kunz TH (1996) Dietary variation in the mexican free-tailed bat (Tadarida brasiliensis mexicana). J Mammal 77:716–724
Wolbert SJ, Zellner AS, Whidden HP (2014) Bat activity, insect biomass, and temperature along an elevational gradient. Northeastern Naturalist 21:72–85
Wordley CFR, Foui EK, Mudappa D et al (2014) Acoustic identification of bats in the Southern Western Ghats, India. Acta Chiropterologica 16:213–222
Wordley CFR, Sankaran M, Mudappa D, Altringham JD (2015) Landscape scale habitat suitability modelling of bats in the western ghats of India: bats like something in their tea. Biol Conserv 191:529–536
Wordley CFR, Sankaran M, Mudappa D, Altringham JD (2017) Bats in the Ghats: Agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biol Conserv 210:48–55
Yao R, Zhao D-D, Zhang S et al (2017) Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest Manag Sci 73:1169–1178
Yuan L, Zhao X, Lin B et al (2011) Adaptive evolution of leptin in heterothermic bats. PLoS ONE 6:e27189–e27189.
Zhao C, Liu B, Piao S et al (2017) Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci USA 114:9326–9331
Zhong-xian LU, Villareal S, Xiao-ping YU et al (2006) Biodiversity and dynamics of planthoppers and their natural enemies in rice fields with different nitrogen regimes. Rice Sci 13:218–226
Zhu S, Malmqvist E, Li W et al (2017) Insect abundance over chinese rice fields in relation to environmental parameters, studied with a polarization-sensitive CW near-IR lidar system. Appl Phys B 123:211.
Acknowledgements
We thank Raju Sharma, Nirmala Devi, Seemanta Jyoti Baishya and Manas Jyoti Talukdar for unwavering support in the field, the Bhagabati family for logistical and emotional succour, Uma Ramakrishnan and Pilot Dovih for bat advice and training, and Rita Banerjee for local coordination. We thank Adrià López-Baucells, Thomas Cherico Wanger, Rohit Chakravarty, and Danielle Linton for advice early in the project. IB thanks his funders, the Rufford Trust (27620-1) and the Rhodes Trust for supporting and funding this project, as well as Jesus College Oxford and the School of Geography and the Environment, University of Oxford, for providing financial support. OR was funded through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/3).
Funding
Iqbal Singh Bhalla was funded by the Rhodes Trust, Jesus College, University of Oxford, and the School of Geography and the Environment, University of Oxford.
Orly Razgour was funded through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/3).
Author information
Authors and Affiliations
Contributions
This manuscript is primarily the work of ISB, who contributed to the study conception and design, data collection, analysis and writing of the manuscript. OR contribution was in the form of advice on bat ecology and behaviour, as well as comments on the drafts of the manuscript. FR contribution was in the form of statistical guidance, comments on the drafts of the manuscript, and small contributions to the code used for the statistical analysis. RW contribution was in the form of guidance on project structure, fieldwork practicalities, comments on the drafts of the manuscript, and general scientific advice. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhalla, I.S., Razgour, O., Rigal, F. et al. Insectivorous bats in Indian rice fields respond to moonlight, temperature, and insect activity. Landsc Ecol 38, 2947–2963 (2023). https://doi.org/10.1007/s10980-023-01764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01764-1