Abstract
Context
Insectivorous bats have been shown to control a number of agricultural insect pests. As bats exhibit species-specific responses to the surrounding landscape, tied closely to their morphology and foraging mode, the activity and distribution patterns of bats, and consequently the ecosystem services they provide, are influenced by the landscape characteristics.
Objectives
This study aims to determine which features in the landscape surrounding rice fields influence the activity levels of insectivorous bats, and at what scales they are most influential.
Methods
We collected acoustic recordings to determine activity levels of seven bat sonotypes in rice fields surrounded by a variety of land-cover types in the Nagaon district of Assam, India. Using this, we determined the most important set of features in the surrounding landscape, and the scales at which had the strongest impact, for each sonotype.
Results
Our results suggest that tree cover variables are the most important predictors of bat activity in rice fields. Distance to nearest forest, area of forest within 1 km, distance to nearest forest edge, and landscape heterogeneity influenced all five of the analysed bat sonotypes. Also important were the amount of urban land within 1 km, which exerted a negative effect on the activity of one sonotype, and moonlight activity, which negatively influenced the activity levels of one sonotype.
Conclusion
Our results demonstrate that when flying over rice fields, bat activity is most influenced by presence and proximity of trees. Therefore, increasing tree cover in agricultural landscapes will increase bat activity and likely the level of pest control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bats (order Chiroptera) are the second most diverse mammalian order, bringing with their diversity substantial ecological and financial value. This includes their potential use as bioindicators (Jones et al. 2009; Russo et al. 2021), and their role as providers of ecosystem services including pest control, soil fertility, seed dispersal, and pollination (Kunz et al. 2011). Of the > 1400 known species of bats (Simmons and Cirranello 2021), nearly two-thirds are insectivores, which thrive in a wide range of habitats—from deserts (Razgour et al. 2018) to wetlands (Mas et al. 2021). In adapting to these environments, bats have evolved highly specialized acoustic and morphological characteristics that enable them to navigate different habitats and hunt prey in a wide variety of conditions (Jones et al. 2016). These same specializations, however, also limit the range of habitats that a species can access. While some bats can easily switch between habitats, others cannot (Denzinger et al. 2016).
Interest in insectivorous bats in agricultural landscapes has risen dramatically in the last two decades, driven in part by the growing evidence of their value as pest suppression agents to crops such as rice (Puig-Montserrat et al. 2015), cotton (Cohen et al. 2020), cacao (Cassano et al. 2016), and corn (Maine and Boyles 2015). Their success as natural pest suppressors comes from a broad diet (Tournayre et al. 2021; Maslo et al. 2022), large energetic demands associated with flight, and, importantly, flexibility in foraging habitat uses. Insectivorous bats quickly switch to the most productive foraging habitat available. When an agricultural plot is fallow between seasons, bats can move to more productive regions elsewhere, to return when there are pest outbreaks (McCracken et al. 2012; Puig-Montserrat et al. 2015).
Agricultural landscapes can provide rich feeding grounds for insectivorous bats, but this relationship is complicated by the seasonal nature of many crops and the species-specific response of bats to the structure and composition of the landscape surrounding agricultural fields. A number of characteristics determine the suitability of a landscape to insectivorous bats. Chief among these is the composition of the wider landscape surrounding the crop fields. Studies of bats in agricultural landscapes consistently find that heterogeneous landscapes support a richer bat community than homogenous ones (Frey-Ehrenbold et al. 2013; Monck-Whipp et al. 2018; Rodríguez-San Pedro et al. 2019) and that higher levels of natural and semi-natural land-cover within agricultural landscapes promote bat activity (Kelly et al. 2016; Kahnonitch et al. 2018). More generally, landscapes that provide bats with access to water bodies support greater bat activity (Heim et al. 2018). Linear elements such as hedgerows and lines of trees constitute key forms of natural land-cover for echolocating bats. These are used both as landmarks for navigation, and for protection against avian predators and wind (Downs and Racey 2006). Even a single row of trees, with insect densities too low to be attractive foraging sites, can be used for commuting and, if the trees are of the right structural form, for roosting (Fischer et al. 2010; Kalda et al. 2015). Increasing the width of the linear features is usually accompanied by larger insect communities, leading to higher bat activity (Russ et al. 2003). As a result, linear features around agricultural fields host bats in greater numbers and diversity than the field interiors do (Kelm et al. 2014; Finch et al. 2020). With many types of agriculture providing less-than-hospitable matrices between forest patches, the presence of suitable linear elements reaching into agricultural fields increases the permeability of such agricultural landscapes (Finch et al. 2020).
The edges between forest and non-forest habitats also provide productive feeding grounds for bats by offering safety from predators. The relative safety and higher foraging success for the generally rich guild of edge-space foragers (Denzinger et al. 2016), results in significantly higher bat activity at edges or in close proximity to linear features compared with open and cluttered habitats (Jantzen and Fenton 2013; Finch et al. 2020). Compounding the requirements for foraging habitats are the requirements for roosting and water. While many bats are capable of commuting long distances to forage, such movements are often along edges. Many bats will avoid open spaces, which bring higher risks of predation, colder temperatures, and faster winds (Downs and Racey 2006). The carrying capacity of a landscape is therefore not solely determined by its composition, but also by the configuration of the elements within it, which shape a bat’s access to food, roosts, and water (Heim et al. 2015). Landscape complementation—whereby close proximity of different landscape elements, and their resulting ease of access, increase the abundance of a species—is particularly evident in bats due to the often contrasting requirements of roosting (in mature trees) and foraging (in more open spaces). A study in Canada found that when the area of different land-cover is kept constant, increased fragmentation led to increased bat activity (Ethier and Fahrig 2011). However, the response of insectivorous bats to fragmentation, in addition to being species-specific, is also heavily dependent on the composition of the matrix. For example, agricultural and production-forests, representing high- and low-contrast matrices respectively, have been shown to provoke opposite reactions to changing forest amount and fragmentation in different bat species (Rodríguez-San Pedro and Simonetti 2015).
In addition to the influence of composition and configuration of landscape features on bat behaviour, their relationship to said features is also influenced by their geographic scale. Morphological traits such as wing shape that allow some bats to travel longer distances than others affect their access to water, roosting sites, and foraging grounds (Meyer et al. 2007; Marinello and Bernard 2014). Adding to this are ecological considerations such as the vulnerability of certain species to avian predators, where some species prefer more closed, and therefore protected, habitats than others (Appel et al. 2021). The same features of a landscape therefore are enabling or limiting to different degrees based on their geographical scale.
Across the world, the intensification and homogenization of agricultural practices (Robinson and Sutherland 2002; Wang et al. 2015) are having severe impacts on bat populations (Park 2015). Given the strong preference of bats for specific landscape structures, changes which reduce tree cover disproportionately affect those species that forage in dense natural vegetation. The general trend shows a greater decline of clutter foragers than open-space foragers with reduced tree cover (Heim et al. 2016; Mtsetfwa et al. 2018).
As agricultural systems worldwide come under increased production pressure, the need for sustainable pest control has bolstered studies of natural pest control measures. Given that bats are valuable pest suppression agents, and that India is striving to increase agricultural production (Hinz et al. 2020), it is important to understand the relationships between bats and agricultural landscapes. This study focusses on the insectivorous bat community in the rice-dominated landscapes of Assam, India. In India, rice makes up 22% of the gross cropped area (Directorate of Economics and Statistics 2019). A small rice farm can offer an ideal habitat for a broad community of insectivorous bats as they feature an abundance of insects (Puig-Montserrat et al. 2015), edges (Harms et al. 2020), roosting sites (in trees and anthropogenic structures) (Kusuminda et al. 2021), and water bodies. As a matrix, rice is a harsh habitat, providing no landmarks, edges, tree cover, or resting sites. The larger the fields are, therefore, the less accessible we expect their interiors to be, as bats foraging in them are then further from water sources, shelter, edges, or roosting sites (Rainho and Palmeirim 2011; Frey-Ehrenbold et al. 2013).
We used passive acoustic recordings from rice fields within the Nagaon district of Assam to investigate the importance and scales at which different landscape features drive the activity of insectivorous bats. We hypothesize that different species of insectivorous bats, will respond differently to presence and proximity of forested areas, treelines, water bodies, grass fields, urban environments and rice fields. In addition to the composition of the landscape, we hypothesize that bat activity will also be influenced its configuration. By identifying how bats use the agricultural landscape, this study can contribute to designing agricultural landscapes for the protection of bats and the promotion of their ecosystem services.
Methods
Study site
The study was conducted in the Nagaon (26.3480° N, 92.6838° E), a district of Assam. Although the typical operational holding of agricultural land in Nagaon (of which rice is the primary crop) is 0.5–1.0 hectares (Saikiam et al. 2020), rice plots usually lie adjacent to one another, so the size of an uninterrupted stretch of rice varies considerably. A typical farming village in Assam has a central road with many branches, dotted on either side with buildings, both concrete and wood. Hubs in a village centre have the highest density of buildings and artificial light, which decrease as one moves further away. Forested or semi-forested vegetation is present in pockets and surrounding most structures. Data for this study came from rice fields surrounding such villages near the city of Nagaon. Nagaon tends to have hot and wet summers. During the months of May and June 2019, when fieldwork took place, the average temperature was 28 °C, with a minimum of 21 °C and a maximum of 35 °C (accessed on 15 Jan 2022 from timeanddate.com). The lack of irrigation leaves many farmers relying on the rains to water their crops (Sharma and Sharma 2015). Historically, the months of May and June see among the highest rainfall of the year, averaging 219 and 311 mm, respectively (accessed on 15 Jan 2022 from timeanddate.com).
Field sampling
We recorded acoustic data using Audiomoth 1.0.0 full spectrum recorders (Hill et al. 2018) with a sampling rate of 384 kHz and a medium gain. Data were collected using six Audiomoth recorders over 18 nights between 9 May and 8 June 2019. A total of 76 sites—all within rice fields—were sampled during this time. The average distance between sites was 13.95 km, but all the sites sampled on a given night were within 1 km of each other (Fig SI–SIII). Sites were chosen for the diversity of the surrounding landscape, which included significant variation in forest area, water bodies, and urban land, as well as the extent of rice fields surrounding the site. Uniform data collection in this landscape proved difficult, and site locations and sampling effort were influenced by logistical, cultural, and safety considerations. As a result, recorders were not always in place by dusk. To account for difference in sampling effort between sites, any recorder that collected less than 90 min of audio in a night was excluded from the analysis, and sampling effort—the number of minutes sampled in a night—was included in the global model of each sonotype.
Acoustic data analysis
The bat calls present in the raw acoustic data, which totalled 481 h, were identified using custom built code in Python version 2.7 (Rossum et al. 1995). The code used to do this can be found at https://github.com/IqbalBhalla/BatClassifier.git.
There are limited acoustic libraries for Indian bats (Wordley et al. 2014), none extending as far as Assam. Without such a library to use as reference, species level identification of recorded calls was not possible, leading us to classify calls into acoustic sonotypes (López-Baucells et al. 2019; Roemer et al. 2021). We determined through manual inspection of the recordings that we had recorded three classes of sonotypes: (i) Constant Frequency (CF), (ii) Frequency modulated—Quasi Constant frequency (FM-QCF), and (iii) Quasi-Constant frequency (QCF). We did not record Pure Frequency Modulated (FM) or FM-CF-FM sonotypes. Within each sonotype class, calls were further classified based on the FMAXE values. These values were determined by our ability to consistently and accurately assign the sample calls into a particular frequency bin. For example, calls that varied between 33 and 35 kHz could not be distinguished from each other, but could consistently be distinguished from those at 31 and 38 kHz. This led to the creation of one sonotype at 31 kHz, one at 34 kHz, and one at 38 kHz. Applying this method to all the calls in our recordings resulted in a total of seven sonotypes: one pure QCF sonotype with an FMAXE of 28 kHz, and six FM-QCF sonotypes with FMAXE values of 20 kHz, 31 kHz, 34 kHz, 38 kHz, 48 kHz, and 65 kHz. While we did record some CF calls, they could not be separated from insect noises, and so were excluded from the analysis. One sonotype contained both QCF and FM-QCF calls within a single pass. This was considered to be an FM-QCF sonotype. What remained were QCF and FM-QCF sonotypes calling at frequencies ranging from 20 to 65 kHz.
Acoustic templates for each of these seven sonotypes were created using manually identified and extracted calls that were representative of the sonotype. Using the MatLab Classification learner, these templates were used to train a classifier based on a linear discriminant analysis (The MathWorks Inc. 2019). This classifier was used to classify all the calls in the raw audio recordings into one of the above seven sonotypes based on the following characteristics: (i) frequency of maximum energy (FMAXE)—the frequency containing the most energy in the call (Wordley et al. 2014); (ii) minimum and maximum frequencies—the lowest and highest frequencies that contained 5% of the energy of FMAXE; (iii) bandwidth—difference in frequency between the minimum and maximum frequencies; (iv) call length—the time interval between the point that the call first crosses 5% FMAXE, and when it last crosses FMAXE; and (v) average amplitude—the average amplitude of the call. All recordings were divided into five-second intervals. One bat pass was defined as an interval containing more than two calls of the same sonotype (Millon et al. 2015).
The classified calls were then processed in R version 3.63 (R Core Team 2020) to reclassify based on FMAXE and bandwidth those few calls that had been misclassified. The resulting dataset was then filtered to remove false positives and bats that were recorded prior to the recorders being put in place. Using a random number generator, 10% of the extracted passes were selected for manual verification. The classifier accurately identified > 90% of the passes.
Spatial data analysis
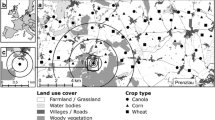
Landscape data used for the analysis came from Level-2 A of Copernicus Sentinel data (2019), accessed on 13 January 2022. Different studies choose different scales to study the influence of landscape structure on bat landscape statistics. These vary based on the objective of the study, the ecology of the bat species in question, and landscape of the study sites. They range between 0.1 and 1 km (Kahnonitch et al. 2018) and 1–5 km around each site (Rodríguez-San Pedro et al. 2019). Due to lack of data on the home and foraging ranges of Indian bats, we chose intermediate scales of analysis for landscape variables, using scales of 500 m, 1000 m, 2000 m, and 3000 m, to account for difference in range sizes between different species. The landscape in a radius of 3 km around every site was classified at a 10 m resolution using a random forest supervised classifier in Google Earth Engine (details can be found in the Supporting Information) (Gorelick et al. 2017). Training data were collected using Google Earth Engine and classified landscapes were verified using Google Earth imagery from January 2019, and knowledge of the field sites. The classification of rice fields was also ground-truthed.
Each 10 × 10 m pixel was classified into one of six landscape types (Fig. 1).

Image source: contains modified Copernicus Sentinel data (2022); Arbus, CNES/Airbus, Landsat/Copernicus, Maxar Technologies, Planet.com. India map source: CC-by-sa PlaneMad/Wikimedia
The location of the sites in the state of Assam, India (left). The landscape classified (right) within a radius of 4 km around the centroid of one cluster of sites (92.67664517498046 E, 26.372194509919872 N) using a random forest supervised classifier in Google Earth Engine into six land-cover types: black dots represent sampling sites. Maps for the remaining sites can be found in the Electronic Supplementary Material 1 (ESM_1)
-
1.
Rice: Pixels containing rice plant.
-
2.
Water: Pixels covered completely with water that was not covered by any algae/plants.
-
3.
Edge: Land filled only partly with trees and partly with a different land cover type.
-
4.
Field: Land with grass but no trees.
-
5.
Forest: Pixels containing only trees.
-
6.
Urban: Area with construction/houses, or roads.
The following types of landscape metrics were calculated for the analysis
(1) Area of all landscape types except water within the four buffers. Size of water bodies was not considered because we used instead distance to the nearest water body as a separate variable due to its higher relevance at the scale of analysis without information on water depth and in a landscape with an abundance of water bodies. (2) Distance of each site to nearest patch of every landscape. Defining a ‘patch’ as 10 or more pixels of a water (equivalent to 50 × 20 m) or 30 or more pixels of the other landscape types (equivalent to 50 × 60 m), the distance to the nearest patch within 3000 m from the recorder was measured. This metric was not calculated for rice because all the recorders were placed in rice fields. (3) Landscape structure metrics. Shannon’s Diversity Index (SHDI) for landscapes was calculated as \(- \sum _{{i = 1}}^{m} \left( {Pi \times \ln \left( {Pi} \right)} \right)\)(Shannon 1948), where ‘i’ is the selected landscape, ‘m’ is the total number of landscapes, and Pi is the proportion of landscape ‘i’ in the selected buffer.
Statistical analysis
Part 1: accounting for spatial autocorrelation
All statistical analyses were performed in R version 3.63 (R Core Team 2020). To maximise the use of the data, while acknowledging and accounting for non-independent data points, data from the 76 sites were grouped in geographical clusters within which data points were considered non-independent. To select the appropriate grouping structure, a distance matrix using the geographic coordinates of all points was created and transformed into a hierarchical cluster tree. Points were then grouped based on a set of scales: 0.1 km, 0.2 km, 0.3 km, 0.4 km, 0.5 km, 1 km, 5 km, 10 and 30 km. At each scale, standardized bat activity—number of passes per unit time—was tested for spatial autocorrelation using the Moran’s I test using the ‘spdep’ R package (Bivand and Wong 2018).
Nightly activity data for each sonotype was tested for spatial autocorrelation at the nine different spatial scales. The smallest scale at which autocorrelation was found to be not significant was used to group the sites and these groups were used as a random effect in the subsequent models.
Part 2: testing the effect of landscape variables on bat activity
The landscape surrounding each site was divided into six landcover types. The area and distance to each landcover type from each site was calculated within buffers of 0.5 km, 1 km, 2 and 3 km, which cover a range of distances commonly used as buffers in studies investigating the influence of landscape characteristics on bat activity (Azam et al. 2016; Falcão et al. 2021; Barré et al. 2022). This resulted in a list of five values for each landcover type. For example, the forest landcover had four area variables—the area of forest within each of the four buffers—and a fifth variable—distance to the nearest forest patch. A generalized linear mixed model (GLMM) was implemented to determine whether area of a particular landcover or distance to the nearest patch of this landcover was the best predictor of activity. Here, and for each sonotype, bat activity—total number of passes recorded over one night—was the response variable. The explanatory variables were sampling effort and one variable of the focal land-cover. Sampling effort was calculated as the number of 10-minute intervals recorded at each site. To account for spatial autocorrelation, the site grouping variable identified in Part 1 was included as a random effect. In addition, we defined an appropriate null model being the GLMM model with intercept only, to highlight a potential absence of relationship. With these data, we constructed one model for each of the variables of a particular landcover type. By comparing these models, we determined which, if any, of the variables—area within one of the four buffers, or distance—was the best predictor of bat activity for that landcover type. The models of every variable of a land cover type and the null model were compared to each other using Akaike’s Information Criterion corrected for sample size (AICc) to determine the most significant variable of a given land-cover type (Bartoń 2020), the best model being the one with the lowest AICc. If the null model performed best, then the land-cover type in question was not taken forward, otherwise the variable included in the best model was incorporated into the global GLMM for that particular sonotype. In the case of rice and Shannon Landscape Diversity Index (SDHI), distance to the nearest patch was not applicable, so only area variables were considered.
The global GLMM was built for each sonotype separately. The response variable was total number of bat passes per sonotype. The explanatory variables included one variable for each landcover type (if found significant in Part 2), ‘moonlight intensity’ (obtained from WorldWeatherOnline.com on 16 August 2021), distance to water, and sampling effort. Sampling effort was calculated as the number of 10-minute intervals recorded at each site. The previously determined site grouping variables were used as the random effect. Prior to running the model, all of the variables were standardized to zero mean and unit variance in order to derive comparable estimates (Schielzeth 2010). Multicollinearity was assessed among all the predictors using Pearson’s correlation with a threshold of r > |0.7| (Dormann et al. 2013). The global GLMM was fitted using the Template Model Builder method for the maximum likelihood estimation with R package ‘glmmTMB’ (Brooks et al. 2017), with a negative binomial distribution parameter to account for over-dispersion (Lindén and Mäntyniemi 2011).
The resulting global model (above) was further reduced to a more parsimonious model including the best set of variables explaining bat activity. This was done by fitting models with all possible combinations of explanatory variables and calculating AICc for each model; the best model being the one with the smallest AICc (Burnham and Anderson 2002). However, all models with a ∆AICc value < 2 (the difference between each model’s AICc and the lowest AICc) were considered as receiving equal statistical support (Burnham and Anderson 2002). In this case, the model with the fewest variables and no obvious pattern in the residuals was chosen as the ‘best model’ (Symonds and Moussalli 2011). Relevant patterns included heteroscedasticity, linear/quadratic trends, and deviance—as measured by the Kolgomorov–Smirnov Test.
The model selection procedure was implemented with the function ‘dredge’ of the package MuMIn (Bartoń 2020) During the procedure, if any variable correlated with another by over a Pearson’s correlation of |0.7|, then a condition was placed on the dredge function to test models with only one of the correlated variables. The residuals of the best models were tested again for spatial autocorrelation at the previously identified scales using moran.test() function. R2 values—the coefficient of determination based on the likelihood ratio test—were calculated using the function r.squaredLR in the MuMIn R package (Bartoń 2020). The absolute values of the standard regression coefficients were rescaled to sum to one to determine the relative importance of each predictor.
Results
A total of 13,263 bat passes belonging to seven sonotypes were isolated, identified, and analysed from 18 nights of recordings. These were divided into seven FM-QCF sonotypes, calling at 20 kHz, 28 kHz, 31 kHz, 34 kHz, 38 kHz, 47 kHz, and 65 kHz, respectively (Table SI). Henceforth, these sonotypes will be referred to using ‘S’ followed by their identifying frequency (e.g. S20). An additional FM-QCF sonotype calling at 18 kHz was identified, but separating calls from insect noise proved challenging and the large number of false positives led to it being dropped from further analysis.
Part 1: accounting for spatial autocorrelation
The smallest scales at which sites were grouped and resulted in no spatial autocorrelation for each sonotype were: S38 and S28 at a scale of 1 km, S35 at a scale of 5 km, S32 at a scale of 0.5 km, and S47 at a scale of 0.4 km. Sonotype S20 did not reach the modelling stage because spatial autocorrelation was detected up to and including a scale of 10 km during Part 1 of the statistical analysis. Grouping data points at a larger scale was deemed redundant and the sonotype was dropped. S65 was also dropped due to insufficient data.
Part 2: the effect of landscape variables on bat activity
Overall, variables indicative of the presence of trees were most prominent and significant in the final models (Fig. 2). Edge variables were present in the final models of S28 and S32. Forest variables were present in the final models of S28, S35, S38. SDHI was only found in the best model of one sonotype—S47. Urban land-cover was present in the best model of two sonotypes, S28 and S38 (Fig. 2a, d). Sampling effort contributed to the best model of all sonotypes. Water, which had only been included in the global model as a distance variable, did not feature in the best model of any sonotype. Similarly, rice, which had only been included in the global model as an area variable, also did not feature in the best model of any sonotype. There was also similarity in the type of variable of each land-cover type that made it into the best model. Edge was only present in the form of distance to edge. Forest was present in the form of distance to forest and forest area within 1 km. Urban was only present as area of urban land-cover within 1 km (Fig. 2). Full details of model results of individual sonotypes can be found in the supporting information (Table SII).
Effect of variables in the best generalized linear mixed models of each sonotype. Panels a–e represent sonotypes S28, S32, S35, S38, and S47. Variables were selected from a global model based on their contribution to significant decrease in AICc values. Dots represent the estimate, solid dots indicate a p-value < 0.05 and hollow dots indicate a p-value > 0.05. Error bars represent the 2.5% and 97.5% confidence interval. The confidence interval around ‘Sampling effort’ is not zero, but is too small for wings to extend beyond the perimeter of the solid dot
In creating a model for overall bat activity, spatial autocorrelation was detected at all relevant scales. For descriptive purposes only, an exploratory set of models were built with activity data of all bat sonotypes combined. Sites were grouped at the 400 m, 1 and 5 km scales. In addition to sampling effort, distance to forest and area of rice within 2 km were the most important variables in the final models, with one model having area of edge within 1 km as a predictor (Table SIII). The similarity of the models, despite having points grouped at different spatial scales, would indicate a relatively low effect of spatial autocorrelation and suggest that these variables do influence bat activity in general.
In considering individual effects, Fig. 3 shows the relationship between bat activity and individual variables present in the final models of all sonotypes. Since the models we built were mixed models, they included random effects that cannot be represented in scatter plots. Nonetheless, these plots are useful for observing patterns in the raw data. Distance to edge and forest, area of urban land, and moonlight intensity (Fig. 3a, c, e–g) represent variables that were statistically significant in the best models of sonotypes S28, S35 and S38. Despite not accounting for the random effect, all of them show a clear negative correlation with bat activity, which is in line with the model estimates and with what other similar studies would predict. Figure 3b, d represent forest area within 1 km (S28) and distance to edge (S32). These are neither statistically significant in the final models (Fig. 2), nor do they show an obvious pattern in scatter plots (Fig. 3b, d). On the other hand, Fig. 3h, i, which represent urban land and SDHI within 1 km for sonotypes S38 and S47, respectively, show clear patterns in the scatter plots, despite being not significant in the final models of these sonotypes. These patterns are also what one would expect based on other similar studies, showing bat activity decreases with an increase in urban land, but increases with an increase in landscape heterogeneity.
Scatter plots of bat activity over all standardized variables present in the final models of each sonotype (except sampling effort). Each panel illustrates the relationship between the activity of one sonotype and one landscape variable. Sonotype S28 in panels a–c, S32 in panel d, S35 in panel e, S38 in panels f–h, and S47 in panel i. Blue dots indicate a p-value < 0.05, salmon dots indicate a p-value > 0.05. These points are raw data, and do not accurately represent the effect of variables in the final models. This is because mixed effect models used in this study included random effects that couldn’t be accounted for in the above scatter plots. Lines and 95% confidence intervals represent the predicted values extracted from the relevant best models
Discussion
We studied bat activity in rice fields of Assam to understand how the wider landscape matrix affects the activity of insectivorous bats and to identify important landscape features for bat conservation in the agricultural landscape. Our results show that key landscape features, including forest and edge cover, urban land, moonlight intensity and landscape heterogeneity influenced the activity of five sonotypes of insectivorous bats flying over rice fields.
In warm and dry environments, bats lose water rapidly (Webb et al. 1995) and it must be replenished through their food and by drinking. While many species have evolved mechanisms to limit water loss (Reher and Dausmann 2021), the presence of water bodies still exert considerable influence on where bats choose to fly, particularly in arid environments (Razgour et al. 2010). Past studies have found distance to water to be a key factor in driving selection of roost sites and foraging grounds (Adams and Thibault 2006; Adams and Hayes 2008; Rainho and Palmeirim 2011). However, water bodies in these studies tend to be few and far apart, increasing their importance to foraging bats. Despite our study being conducted in the summer, when temperatures regularly exceeded 30 °C, having been conducted in rice fields, our sites were all within 600 m of a water body (defined here as areas of water of at least 1000 sq meters, equivalent to a 50 m by 20 m plot), with an average distance of 204 m. Given that this distance is well within the foraging ranges of insectivorous bats, water was not found to be an influencing factor in the best model of any sonotype, suggesting that for the analysed sonotypes, water is either not a limiting factor in this landscape, or the range at which water becomes a limiting factor is greater than bats in our landscapes were presented with.
Urban landscapes are not entirely uninhabitable, and many bat species have adapted to roost and forage there. The use of urban land by bats is influenced by built infrastructure, light pollution, noise levels, tree cover, bat physiology, predation pressure, and prey availability (Moretto and Francis 2017; Moretto et al. 2019; Jung and Threlfall 2021). While light pollution in urban areas is harmful to insect populations at the large scale (Owens et al. 2020), the insects attracted to streetlights create local zones of high prey density (Firebaugh and Haynes 2019), which in turn can increase the activity of some bats (Rodríguez-Aguilar et al. 2017). Bat activity is greater when urban areas are near patches of forest, water, and/or agricultural land-cover (Dixon 2012; Ancillotto et al. 2019), in part because of higher insect activity in such areas (Avila-Flores and Fenton 2005). Studies have found that the complementation of anthropogenic and natural land-cover can result in high levels of bat activity, particularly of mobile generalist species (Johnson et al. 2008). More generally, canopy cover has been found to be a key determinant of bat activity in urban contexts (Bailey et al. 2019). Bats exhibit extremely species-specific responses to landscapes and while some studies have found increased activity of bats in urban landscapes (Rodríguez-Aguilar et al. 2017), many studies have found even moderate urbanization to have negative impacts on bat activity (Ancillotto 2015; Jung and Threlfall 2016). While our sites were set in rice fields, urban land-cover was present around the sites in two forms: dense urban landscapes seen in Nagaon city and scattered nodes in villages. One, or both, of these land-covers of these negatively affected the activity of S28 and S38. For the other sonotypes, it is possible that the farmland and associated buildings, trees, and water bodies satisfied the requirements of most bats at the local scale, thereby overriding any negative (or positive) effects of the city.
Forests and forest boundaries, including linear features such as tree lines, are important habitats for bats (Heim et al. 2015). However, separating the often concurrent reasons for using such habitats, including roosting, safety against predators (Heim et al. 2018) and protection from the wind (Verboom and Huitema 1997), landmarks for navigation, and improved foraging (Jantzen and Fenton 2013), can be a challenge. Some of these benefits change with time. For example, brighter nights allow predators to see better (Prugh and Golden 2014), increasing the importance of sheltered habitats. Similarly, prey abundance in forests relative to the surrounding landscape varies with season, particularly when they are adjacent to agricultural landscapes (Noer et al. 2012; Davidai et al. 2015). On the other hand, the importance of trees as roosting sites remains more consistent over time.
Prey availability is a strong driver of bat activity and while it is difficult to compare the activity of different bats in different settings, studies have found that insectivorous bats do, in general, consider in-season rice fields to be prey-rich habitat (Sedlock et al. 2019; Toffoli and Rughetti 2020). Activity is increased by the presence of forest patches nearby (Heim et al. 2015; Bailey et al. 2019). Some studies have shown insectivorous bats to preferentially hunt over rice fields compared to forested areas (Puig-Montserrat et al. 2015; Kemp et al. 2019; Katunzi et al. 2021). Other studies report that while rice fields were attractive foraging grounds, nearby natural wetlands saw higher levels of foraging activity (Toffoli and Rughetti 2017).
While the edge variables calculated and tested for in this study included area of forest edge (within various buffers from the focal site), only distance to the nearest edge was retained in the best models of S28 and S32. The activity of sonotypes S35 and S38 was negatively influenced by the distance to the nearest forest patch while S28 was positively influenced by the area of forest within 1 km. We hypothesize that forests and forest edges surrounding rice fields containing our sites were attractive for safety or the presence of roost sites. This is augmented by the fact that the bamboo houses and sheds in villages were known to be used as roost sites (IB, systematic inspection). These structures, being smaller than concrete buildings, more enclosed by trees, and bearing thatched roofs, were usually classified as ‘edge’ rather than ‘urban’ land-cover. This adds to the argument that ‘edges’, as classified in this study, also represented roost sites to bats in the study area.
Area of rice was not present in the best models of any sonotype. The most likely reason being that since our sites were all in rice fields, set in rice-dominated landscapes, prey availability was great enough that larger areas did not improve foraging success through reduced competition or increased prey availability. Rice-dominated landscapes present a harsh matrix interspersed with patches of forest-urban-edge combinations that provide foraging grounds, roost sites, and relative safety. Harsher (more contrasting) matrices evoke stronger reactions to fragmented landscapes (Rodríguez-San Pedro and Simonetti 2015; Farneda et al. 2020) and unbroken rice fields provide little by way of shelter or roost sites. Greater landscape heterogeneity increases the proximity of different land-cover types. This can result in larger bat populations and higher bat activity because bats that roost and forage in different landscapes have easy access to both, reducing potentially commuting costs (Ethier and Fahrig 2011). An increase in landscape heterogeneity also correlates with availability of edges between natural and agricultural land-cover. These interfaces are important for edge-space foragers that avoid open spaces either because they are more vulnerable to predators in open spaces, or because they have a higher foraging success at edges (Lentini et al. 2012; Frey-Ehrenbold et al. 2013). Greater heterogeneity also increases insect populations by providing spaces for them to breed and survive fallow seasons (Sigsgaard 2000; Fahrig et al. 2015; Bertrand et al. 2016; Chaperon et al. 2022). Sonotype S47 was influenced by landscape heterogeneity, most strongly at the scale of 1 km (Fig. 2), supporting other studies that demonstrate the positive effect of landscape heterogeneity on bat activity (Frey-Ehrenbold et al. 2013; Monck-Whipp et al. 2018; Rodríguez-San Pedro et al. 2019).
Agricultural industries globally face an enormous challenge to reduce their reliance on environmentally unsustainable practices, such as the use of chemical pesticides, for both environmental and financial reasons. Pests are predicted to cause increasing losses to many major crops (Deutsch et al. 2018) and chemical methods of control, far from eliminating pests, have often prompted the emergence of more resistant strains (Normile 2013). The effective use of natural enemies as a sustainable alternative is only practised in pockets around the world (Lou et al. 2013; Puig-Montserrat et al. 2015), but holds great potential to alleviate the dual burdens of declining agricultural and ecological health. Realizing this potential requires both an understanding of the ecological relationships that underpin these ecosystem services and of the ecology of the service providers, in order to make agricultural landscapes more habitable to them. Previous studies have managed to artificially increased bat populations and seen significant improvement in insect pest control as a result (Puig-Montserrat et al. 2015). However, bats do have an unusually slow reproductive rate (Barclay and Harder 2003) which limits the practicality of designing natural pest control measures primarily dependant on insectivorous bats.
Studies of bats in agricultural landscapes have shown the need for connectivity, heterogeneity, and the presence of natural land-cover (Downs and Racey 2006; Frey-Ehrenbold et al. 2013; Finch et al. 2020). Few have considered the rice landscape, and none hitherto in India. This study demonstrated that in rice landscapes of Assam, while proximity to urban land decreased bat activity, the presence of forest patches and edges, and higher levels of landscape heterogeneity promoted bat activity, and likely by extension also increased the control of insect pests.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adams RA, Hayes MA (2008) Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J Anim Ecol 77:1115–1121.
Adams RA, Thibault KM (2006) Temporal resource partitioning by bats at water holes. J Zool 270:466–472.
Ancillotto L (2015) Sensitivity of bats to urbanization: a review. Mamm Biol 80:205–212
Ancillotto L, Bosso L, Salinas-Ramos VB, Russo D (2019) The importance of ponds for the conservation of bats in urban landscapes. Landsc Urban Plan 190:103607
Appel G, López-Baucells A, Rocha R et al (2021) Habitat disturbance trumps moonlight effects on the activity of tropical insectivorous bats. Anim Conserv 24:1046–1058.
Avila-Flores R, Fenton MB (2005) Use of spatial features by foraging insectivorous bats in a large urban landscape. J Mamm 86:1193–1204
Azam C, Le Viol I, Julien J-F et al (2016) Disentangling the relative effect of light pollution, impervious surfaces and intensive agriculture on bat activity with a national-scale monitoring program. Landscape Ecol 31:2471–2483.
Bailey AM, Ober HK, Reichert BE, McCleery RA (2019) Canopy cover shapes bat diversity across an urban and agricultural landscape mosaic. Environ Conserv 46:193–200.
Barclay RM, Harder LD (2003) Life histories of bats: life in the slow lane. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, p 253
Barré K, Vernet A, Azam C et al (2022) Landscape composition drives the impacts of artificial light at night on insectivorous bats. Environ Pollut 292:118394.
Bartoń K (2020) MuMIn: multi-model inference. https://CRAN.R-project.org/package=MuMIn
Bertrand C, Burel F, Baudry J (2016) Spatial and temporal heterogeneity of the crop mosaic influences carabid beetles in agricultural landscapes. Landsc Ecol 31:451–466
Bivand RS, Wong DWS (2018) Comparing implementations of global and local indicators of spatial association. Test 27:716–748.
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400.
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cassano CR, Silva RM, Mariano-Neto E et al (2016) Bat and bird exclusion but not shade cover influence arthropod abundance and cocoa leaf consumption in agroforestry landscape in northeast Brazil. Agric Ecosyst Environ 232:247–253.
Chaperon PN, Rodríguez-San Pedro A, Beltrán CA et al (2022) Effects of adjacent habitat on nocturnal flying insects in vineyards and implications for bat foraging. Agric Ecosyst Environ 326:107780.
Cohen Y, Bar-David S, Nielsen M et al (2020) An appetite for pests: synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol Ecol 29:1185–1198.
Davidai N, Westbrook JK, Lessard J-P et al (2015) The importance of natural habitats to brazilian free-tailed bats in intensive agricultural landscapes in the Winter Garden region of Texas, United States. Biol Conserv 190:107–114.
Denzinger A, Kalko EKV, Tschapka M et al (2016) Guild structure and niche differentiation in echolocating bats. In: Fenton MB, Grinnell AD, Popper AN, Fay RR (eds) Bat Bioacoustics. Springer, New York, pp 141–166
Deutsch CA, Tewksbury JJ, Tigchelaar M et al (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919.
Directorate of Economics and Statistics (2019) Agricultural statistics at a glance 2019. Ministry of Agriculture, Government of India
Dixon MD (2012) Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosyst 15:683–695.
Dormann CF, Elith J, Bacher S et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46.
Downs NC, Racey PA (2006) The use by bats of habitat features in mixed farmland in Scotland. Acta Chiropterologica 8:169–185.
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc Ecol 26:865–876
Fahrig L, Girard J, Duro D et al (2015) Farmlands with smaller crop fields have higher within-field biodiversity. Agric Ecosyst Environ 200:219–234.
Falcão F, Dodonov P, Caselli CB et al (2021) Landscape structure shapes activity levels and composition of aerial insectivorous bats at different spatial scales. Biodivers Conserv 30:2545–2564.
Farneda FZ, Meyer CFJ, Grelle CEV (2020) Effects of land-use change on functional and taxonomic diversity of neotropical bats. Biotropica 52:120–128.
Finch D, Schofield H, Mathews F (2020) Habitat associations of bats in an agricultural landscape: linear features versus open habitats. Animals 10:1856
Firebaugh A, Haynes KJ (2019) Light pollution may create demographic traps for nocturnal insects. Basic Appl Ecol 34:118–125.
Fischer J, Stott J, Law BS (2010) The disproportionate value of scattered trees. Biol Conserv 143:1564–1567.
Frey-Ehrenbold A, Bontadina F, Arlettaz R, Obrist MK (2013) Landscape connectivity, habitat structure and activity of bat guilds in farmland-dominated matrices. J Appl Ecol 50:252–261.
Gorelick N, Hancher M, Dixon M et al (2017) Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27.
Harms K, Omondi E, Mukherjee A (2020) Investigating bat activity in various agricultural landscapes in northeastern United States. Sustainability 12:1959
Heim O, Treitler JT, Tschapka M et al (2015) The importance of landscape elements for bat activity and species richness in agricultural areas. PLoS ONE 10:e0134443–e0134443.
Heim O, Schröder A, Eccard J et al (2016) Seasonal activity patterns of european bats above intensively used farmland. Agric Ecosyst Environ 233:130–139.
Heim O, Lenski J, Schulze J et al (2018) The relevance of vegetation structures and small water bodies for bats foraging above farmland. Basic Appl Ecol 27:9–19.
Hill AP, Prince P, Piña Covarrubias E et al (2018) AudioMoth: evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods Ecol Evol 9:1199–1211.
Hinz R, Sulser TB, Huefner R et al (2020) Agricultural development and land use change in India: a scenario analysis of trade-offs between UN sustainable development goals (SDGs). Earth’s Future 8:e2019EF001287
Jantzen MK, Fenton MB (2013) The depth of edge influence among insectivorous bats at forest–field interfaces. Can J Zool 91:287–292.
Johnson J, Gates J, Ford W (2008) Distribution and activity of bats at local and landscape scales within a rural–urban gradient. Urban Ecosyst 11:227–242.
Jones G, Jacobs DS, Kunz TH et al (2009) Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8:93–115.
Jones PL, Page RA, Ratcliffe JM (2016) To scream or to listen? Prey detection and discrimination in animal-eating Bats BT. In: Fenton MB, Grinnell AD, Popper AN, Fay RR (eds) Bat Bioacoustics. Springer, New York, pp 93–116
Jung K, Threlfall CG (2016) Urbanisation and its Effects on Bats—a global Meta-analysis. Bats in the Anthropocene: conservation of bats in a changing World. Springer, Cham, pp 13–33
Jung K, Threlfall CG (2021) Trait-dependent tolerance of bats to urbanization: a global meta-analysis. Proc Royal Soc B: Biol Sci 285:20181222
Kahnonitch I, Lubin Y, Korine C (2018) Insectivorous bats in semi-arid agroecosystems—effects on foraging activity and implications for insect pest control. Agric Ecosyst Environ 261:80–92
Kalda O, Kalda R, Liira J (2015) Multi-scale ecology of insectivorous bats in agricultural landscapes. Agric Ecosyst Environ 199:105–113.
Katunzi T, Soisook P, Webala PW et al (2021) Bat activity and species richness in different land-use types in and around Chome Nature Forest Reserve, Tanzania. Afr J Ecol 59:117–131.
Kelly RM, Kitzes J, Wilson H, Merenlender A (2016) Habitat diversity promotes bat activity in a vineyard landscape. Agric Ecosyst Environ 223:175–181.
Kelm DH, Lenski J, Kelm V et al (2014) Seasonal bat activity in relation to distance to hedgerows in an agricultural landscape in Central Europe and implications for wind energy development. Acta Chiropterologica 16:65–73.
Kemp J, López-Baucells A, Rocha R et al (2019) Bats as potential suppressors of multiple agricultural pests: a case study from Madagascar. Agric Ecosyst Environ 269:88–96.
Kunz TH, Braun de Torrez E, Bauer D et al (2011) Ecosystem services provided by bats. Ann NY Acad Sci 1223:1–38
Kusuminda T, Mannakkara A, Gamage R et al (2021) Roosting ecology of insectivorous bats in a tropical agricultural landscape. Mammalia. https://doi.org/10.1515/mammalia-2021-0056
Lentini PE, Gibbons P, Fischer J et al (2012) Bats in a farming landscape benefit from linear remnants and unimproved pastures. PLoS ONE 7:e48201.
Lindén A, Mäntyniemi S (2011) Using negative binomial distribution to model overdispersion in ecological count data. Ecology 92:1414–1421.
López-Baucells A, Torrent L, Rocha R et al (2019) Stronger together: combining automated classifiers with manual post-validation optimizes the workload vs reliability trade-off of species identification in bat acoustic surveys. Ecol Inf 49:45–53.
Lou Y-G, Zhang G-R, Zhang W-Q et al (2013) Biological control of rice insect pests in China. Biol Control 67:8–20.
Maine JJ, Boyles JG (2015) Bats initiate vital agroecological interactions in corn. Proc Natl Acad Sci USA 112:12438–12443
Marinello MM, Bernard E (2014) Wing morphology of neotropical bats: a quantitative and qualitative analysis with implications for habitat use. Can J Zool 92:141–147.
Mas M, Flaquer C, Rebelo H, López-Baucells A (2021) Bats and wetlands: synthesising gaps in current knowledge and future opportunities for conservation. Mammal Rev 51:369–384.
Maslo B, Mau RL, Kerwin K et al (2022) Bats provide a critical ecosystem service by consuming a large diversity of agricultural pest insects. Agric Ecosyst Environ 324:107722.
McCracken GF, Westbrook JK, Brown VA et al (2012) Bats track and exploit changes in insect pest populations. PLoS ONE 7:e43839–e43839.
Meyer CFJ, Fründ J, Lizano WP, Kalko EKV (2007) Ecological correlates of vulnerability to fragmentation in neotropical bats. J Appl Ecol 45:381–391.
Millon L, Julien J-F, Julliard R, Kerbiriou C (2015) Bat activity in intensively farmed landscapes with wind turbines and offset measures. Ecol Eng 75:250–257.
Monck-Whipp L, Martin AE, Francis CM, Fahrig L (2018) Farmland heterogeneity benefits bats in agricultural landscapes. Agric Ecosyst Environ 253:131–139.
Moretto L, Francis CM (2017) What factors limit bat abundance and diversity in temperate, north american urban environments? J Urban Ecol 3:1.
Moretto L, Fahrig L, Smith AC, Francis CM (2019) A small-scale response of urban bat activity to tree cover. Urban Ecosyst 22:795–805.
Mtsetfwa F, McCleery RA, Monadjem A (2018) Changes in bat community composition and activity patterns across a conservation-agriculture boundary. Afr Zool 53:99–106.
Noer CL, Dabelsteen T, Bohmann K, Monadjem A (2012) Molossid bats in an african agro-ecosystem select sugarcane fields as foraging habitat. Afr Zool 47:1–11.
Normile D (2013) Vietnam turns back a tsunami of pesticides. Science 341:737–738.
Owens ACS, Cochard P, Durrant J et al (2020) Light pollution is a driver of insect declines. Biol Conserv 241:108259.
Park KJ (2015) Mitigating the impacts of agriculture on biodiversity: bats and their potential role as bioindicators. Mamm Biol 80:191–204
Prugh LR, Golden CD (2014) Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J Anim Ecol 83:504–514.
Puig-Montserrat X, Torre I, López-Baucells A et al (2015) Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mamm Biol 80:237–245
Rainho A, Palmeirim JM (2011) The importance of distance to resources in the spatial modelling of bat foraging habitat. PLoS ONE 6:e19227.
Razgour O, Korine C, Saltz D (2010) Pond characteristics as determinants of species diversity and community composition in desert bats. Anim Conserv 13:505–513.
Razgour O, Persey M, Shamir U, Korine C (2018) The role of climate, water and biotic interactions in shaping biodiversity patterns in arid environments across spatial scales. Divers Distrib 24:1440–1452.
R Core Team (2020) R: A language and environment for statistical computing
Reher S, Dausmann KH (2021) Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc Royal Soc B: Biol Sci 288:20202059
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176
Rodríguez-Aguilar G, Orozco-Lugo CL, Vleut I, Vazquez L-B (2017) Influence of urbanization on the occurrence and activity of aerial insectivorous bats. Urban Ecosyst 20:477–488.
Rodríguez-San Pedro A, Simonetti JA (2015) The relative influence of forest loss and fragmentation on insectivorous bats: does the type of matrix matter? Landscape Ecol 30:1561–1572.
Rodríguez-San Pedro A, Rodríguez-Herbach C, Allendes JL et al (2019) Responses of aerial insectivorous bats to landscape composition and heterogeneity in organic vineyards. Agric Ecosyst Environ 277:74–82.
Roemer C, Julien J-F, Bas Y (2021) An automatic classifier of bat sonotypes around the world. Methods Ecol Evol 12:2432–2444.
Van Rossum G Jr, Drake FL (1995) Python reference manual. Centrum voor Wiskunde en Informatica, Amsterdam
Russ JM, Briffa M, Montgomery WI (2003) Seasonal patterns in activity and habitat use by bats (Pipistrellus spp. and Nyctalus leisleri) in Northern Ireland, determined using a driven transect. J Zool 259:289–299.
Russo D, Salinas-Ramos VB, Cistrone L et al (2021) Do we need to use bats as bioindicators? Biology 10:693.
Saikiam K, Saikia S, Medhi B (2020) Changing trends of landuse landcover in Nagaon district and its impact on agricultural and environmental sustainability. Eur J Mol Clin Med 7:1388–1410
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113.
Sedlock J, Stuart A, Horgan F et al (2019) Local-scale bat guild activity differs with rice growth stage at ground level in the Philippines. Diversity 11:148.
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423.
Sharma B, Sharma H (2015) Status of rice production in Assam, India. J Rice Res: Open Access 3:e121
Sigsgaard L (2000) Early season natural biological control of insect pests in rice by spiders-and some factors in the management of the cropping system that may affect this control. Eur Arachnol 2000:57–64
Simmons NB, Cirranello AL (2021) Bat Species of the World: A taxonomic and geographic database. https://batnames.org/. Accessed 16 Dec 2021
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21.
The MathWorks Inc (2019) MATLAB and Statistics Toolbox Release 2019b. Natick, Massachusetts
Toffoli R, Rughetti M (2017) Bat activity in rice paddies: Organic and conventional farms compared to unmanaged habitat. Agric Ecosyst Environ 249:123–129.
Toffoli R, Rughetti M (2020) Effect of water management on bat activity in rice paddies. Paddy Water Environ 18:687–695.
Tournayre O, Leuchtmann M, Galan M et al (2021) eDNA metabarcoding reveals a core and secondary diets of the greater horseshoe bat with strong spatio-temporal plasticity. Environ DNA 3:277–296.
Verboom B, Huitema H (1997) The importance of linear landscape elements for the pipistrelle Pipistrellus pipistrellus and the serotine bat Eptesicus serotinus. Landscape Ecol 12:117–125.
Wang J, Chen KZ, Gupta S, Das, Huang Z (2015) Is small still beautiful? A comparative study of rice farm size and productivity in China and India. China Agricultural Economic Review 7:484–509.
Webb PI, Speakman JR, Racey PA (1995) Evaporative water loss in two sympatric species of vespertilionid bat, Plecotus auritus and Myotis daubentoni: relation to foraging mode and implications for roost site selection. J Zool 235:269–278.
Wordley CFR, Foui EK, Mudappa D et al (2014) Acoustic identification of bats in the Southern Western Ghats, India. Acta Chiropterologica 16:213–222.
Acknowledgements
We are grateful to Dr. Smarjit Ojah, Monish Thappa, Mr. Sanjib Goswami, and Snigdha Das for invaluable support throughout the fieldwork. We also thank the Divisional Forest Officer, Nagaon District for supporting and encouraging the work and the farmers who welcomed Bhalla into their homes. Bhalla thanks his funders, the Rhodes Trust for supporting and funding this project, as well as Jesus College, University of Oxford, and the School of Geography and the Environment, University of Oxford, for providing financial support. Razgour was funded through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/3).
Funding
Iqbal Singh Bhalla was funded by the Rhodes Trust, Jesus College, University of Oxford, and the School of Geography and the Environment, University of Oxford. Orly Razgour was funded through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/3).
Author information
Authors and Affiliations
Contributions
This manuscript is primarily the work of ISB, who contributed to the study conception and design, data collection, analysis and writing of the manuscript. OR contribution was in the form of advice on bat ecology and behaviour, as well as comments on the drafts of the manuscript. FR contribution was in the form of statistical guidance, comments on the drafts of the manuscript, and small contributions to the code used for the statistical analysis. RW contribution was in the form of guidance on project structure, fieldwork practicalities, comments on the drafts of the manuscript, and general scientific advice. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhalla, I.S., Razgour, O., Rigal, F. et al. Landscape features drive insectivorous bat activity in Indian rice fields. Landsc Ecol 38, 2931–2946 (2023). https://doi.org/10.1007/s10980-023-01758-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01758-z