Abstract
Context
Functional connectivity is vital for plant species dispersal, but little is known about how habitat loss and the presence of green infrastructure interact to affect both functional and structural connectivity, and the impacts of each on species groups.
Objectives
We investigate how changes in the spatial configuration of species-rich grasslands and related green infrastructure such as road verges, hedgerows and forest borders in three European countries have influenced landscape connectivity, and the effects on grassland plant biodiversity.
Methods
We mapped past and present land use for 36 landscapes in Belgium, Germany and Sweden, to estimate connectivity based on simple habitat spatial configuration (structural connectivity) and accounting for effective dispersal and establishment (functional connectivity) around focal grasslands. We used the resulting measures of landscape change to interpret patterns in plant communities.
Results
Increased presence of landscape connecting elements could not compensate for large scale losses of grassland area resulting in substantial declines in structural and functional connectivity. Generalist species were negatively affected by connectivity, and responded most strongly to structural connectivity, while functional connectivity determined the occurrence of grassland specialists in focal grasslands. Restored patches had more generalist species, and a lower density of grassland specialist species than ancient patches.
Conclusions
Protecting both species rich grasslands and dispersal pathways within landscapes is essential for maintaining grassland biodiversity. Our results show that increases in green infrastructure have not been sufficient to offset loss of semi-natural habitat, and that landscape links must be functionally effective in order to contribute to grassland diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss due to land use change is a key driver of global plant biodiversity declines (Foley et al. 2005; Newbold et al. 2015; Auffret et al. 2018). Grassland communities are particularly threatened because their high biodiversity depends on increasingly rare grazing management, along with high connectivity (Wilson et al. 2012; Cousins et al. 2015; Plue and Cousins 2018). Much of Europe has experienced an ongoing loss of species-rich grasslands over the last century, with many actively afforested, converted to arable land or intensive grassland, or abandoned to passively become forest (Eriksson et al. 2002; WallisDeVries et al. 2002; Kuemmerle et al. 2016; Watson et al. 2016). This habitat loss often leads to a decline in landscape connectivity for grassland plants (Hooftman and Bullock 2012; Auffret et al. 2015; Cousins et al. 2015).
Loss of landscape connectivity, i.e. reductions in the extent to which the landscape facilitates the movement of species (Taylor et al. 1993; Auffret et al. 2017) threatens grassland biodiversity, since plant populations in small grassland fragments are less likely to be rescued from local extinction through immigration from neighbouring populations (Eriksson 1996; Evju et al. 2015; Hooftman et al. 2015; Aguilar et al. 2019; Damschen et al. 2019). This is particularly the case for species with low dispersal capability and in sites which are no longer rotationally grazed by animals moving between habitat areas (Römermann et al. 2008; Ozinga et al. 2009; Schleicher et al. 2011; Plue et al. 2019). Declines in connectivity may also reduce our ability to restore species-rich grasslands on former agricultural or abandoned areas. Restored grasslands recover biodiversity more quickly when more species are present within the wider landscape, and when the sites are well-connected to other grassland habitats (Poschlod et al. 1998; Fagan et al. 2008; Piqueray et al. 2011, 2015; Winsa et al. 2015; Waldén et al. 2017).
Landscape connectivity is typically considered in terms of the physical amount and spatial distribution of suitable habitat, i.e. the structural connectivity (Haddad et al. 2015; McGuire et al. 2016). However, the functional connectivity of a landscape may be more ecologically meaningful, as it represents the ability of species to disperse effectively among habitat patches (Auffret et al. 2017). Functional connectivity is highly dependent upon structural connectivity, but also encompasses the ability of plant species to move among, and successfully establish within, suitable patches. Hence, it is also determined by the quality of available habitat and the behaviour and abundance of important biotic dispersal vectors such as birds, humans, or grazing livestock (Tischendorf and Fahrig 2000; Auffret et al. 2017). As well as declines in structural connectivity through direct habitat loss, land use intensification and the abandonment of traditional rotational grazing networks has likely further disrupted grassland functional connectivity by reducing the potential for plant species to disperse through stepping stones within the landscape and via livestock vectors (Römermann et al. 2008; Auffret and Cousins 2013; Plue and Cousins 2018).
Within a fragmented landscape, small natural features can form part of a network of “green infrastructure” and positively contribute to landscape connectivity. Although green infrastructure is a broad term, it is widely used in environmental policy (e.g. the European Union Strategy on Green Infrastructure https://ec.europa.eu/environment/nature/ecosystems/strategy/index_en.htm). It can be defined as a network of core habitat and other features that might support biodiversity and ecosystem services at the landscape scale (Garmendia et al. 2016; Bullock et al. 2018). As such, the definition of these green infrastructure habitats relevant to semi-natural grassland connectivity includes restored grasslands, forest borders, midfield islets (small, often rocky areas with a thin topsoil layer within crop fields), managed hedgerows and road verges. Although these landscape features do not have all the environmental attributes of ancient semi-natural grassland, they may support at least some populations of semi-natural grassland species (Cousins 2006; Auffret and Cousins 2013; Jakobsson et al. 2016; Hunter et al. 2017; Poschlod and Braun-Reichert 2017; Lindgren et al. 2018; Thiele et al. 2018). Hence, green infrastructure habitats may enhance both structural and functional connectivity by increasing available habitat and linking otherwise isolated grasslands through supporting dispersal processes or the movement of dispersal vectors through the landscape (Auffret and Cousins 2013; Hunter et al. 2017; Poschlod and Braun-Reichert 2017; Bullock et al. 2018; Damschen et al. 2019). Given the loss of species-rich grassland across much of Europe, such green infrastructure may therefore provide important additional functional connectivity (Bullock et al. 2018). However, the contribution of this green infrastructure to landscape connectivity, particularly for specialist grassland species that may be more restricted to core semi-natural grassland areas and heavily dependent on dispersal vectors such as livestock, remains unclear (Plue et al. 2019). Likewise, while road verges, hedgerows and forest borders might be structurally connecting elements, their role in providing both structural and functional connectivity will depend on species’ dispersal abilities and ability to establish in these habitat types (van Dijk et al. 2014).
Understanding how land use change has affected structural and functional connectivity and the impacts on grassland plant communities is key to understanding how contemporary rural landscapes can be managed to conserve biodiversity (Lindborg and Eriksson 2004; Cousins et al. 2015). Here, using landscapes in three European countries, we assess changes in land use composition that have occurred over the last 50 years in terms of both semi-natural grassland loss and changes in green infrastructure. We then quantify how resulting changes in landscape composition have affected landscape connectivity, using resistance surfaces that estimate both structural and functional connectivity. We expect to see a decline in grassland area across all regions, accompanied by an associated decline in landscape connectivity, and particularly in functional connectivity. Finally, we investigate the ability of structural and inferred functional connectivity to explain variation in grassland plant communities, in terms of grassland specialist species and more generalist species, in both restored and ancient grasslands. Grassland patches are expected to have higher total (gamma) diversity where they are embedded in high connectivity landscapes, due to the greater numbers of species able to reach sites via spatial dispersal (Baur 2014: Auffret et al. 2018). Greater habitat availability and connectivity within the landscape also enables species to develop larger populations and to occupy a greater proportion of available microsites (Erikson, 1996). Higher levels of connectivity are therefore also expected to increase smaller-scale species richness within patches (alpha diversity), and reduce turnover of species (beta diversity) between different parts of grasslands. This may, however, depend on an interaction between connectivity and grassland age, because more recently restored grasslands may not have had enough time to develop the high density of species typical of ancient grassland habitats (Schmid et al. 2017; Damschen et al. 2019). Grassland specialists, which are likely to be less able to utilise structural connecting elements, should be more dependent on functional connectivity and on the age of grassland sites (Evju et al. 2015). Conversely, generalist species which may benefit from landscape linear and remnant features may be more closely related to landscape structural connectivity, and be less influenced by the history of grasslands.
Methods
Study regions
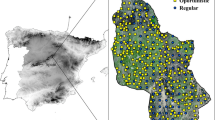
The grassland landscapes were situated in three European countries, Belgium (Viroin region), Germany (Regensburg county, Kallmünz region) and Sweden (Södermanland county and the Stockholm archipelago). In each of these three regions, we selected 12 focal semi-natural grassland patches, six ancient (continuously managed through grazing for centuries) and six restored (abandoned at some point in the past but with grazing management recently re-established) (Adriaens et al. 2006; Poschlod et al. 2008). These were chosen to cover landscapes with a range of grassland and other semi-natural habitats, and therefore represent a gradient of present-day connectivity for grassland plants. Size of the focal grasslands ranged from 0.28 ha to 5.85 ha. All focal grasslands were subject to grazing management, by sheep or cattle. Restored grasslands were mostly restored by removal of successional scrub and tree growth on abandoned pasture, but some sites (mostly in Germany) were restored onto former arable fields.
Connectivity data
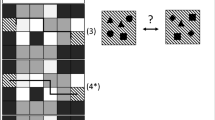
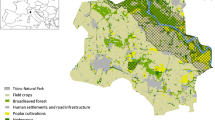
Our approach to assessing connectivity and its role in these landscapes involved determining metrics representing both structural and inferred functional connectivity, determining how these have changed in each landscape, and assessing the ability of each metric to explain variation in species composition in the focal grasslands. We digitised land cover within a 1600 m buffer drawn from the centroid of each focal grassland. Grassland species composition has been shown to be related to landscape composition over similar distances in the past (e.g. Adriaens et al. 2006). Digitisation was performed for past time periods using black and white aerial photographs (from 1965 in Belgium, 1952–1963 in Germany and 1952 in Sweden) and present time periods using colour aerial orthophotographs (from 2015 in Belgium and 2017 in Germany and Sweden). Past dates represented the earliest time period for which consistent historical landscape information could be obtained for all three regions. Land classes identified in historical aerial photographs were arable land, water, built-up land, open grassland, semi-open grazed forest (hereafter open forest), midfield islets (small, often dry or rocky areas contained in arable fields (Cousins 2006)), wetland and dense, closed forest. We identified the same classes for present day landscapes, with the dense forest class split into deciduous, coniferous and logged forest (this was not possible for earlier black and white photographs). No semi-open grazed forest remained in contemporary landscapes, so this category was not present in the contemporary landscape digitization. We also digitised road verges, hedges, railway banks and complex forest borders (forest borders where the transition from open to forest habitat is gradual, resulting in heterogeneous conditions with some more open areas (Lindgren et al. 2018)), for both time points.
We considered semi-natural grassland, open forest, midfield islets, forest borders and road verges as potential “green infrastructure” (GI) habitats for grassland plant species, in relation to our focal habitats (Cousins 2006; Poschlod and Braun-Reichert 2017; Lindgren et al. 2018). We created two metrics of connectivity following Hanski (1994), which we adapted to represent structural and inferred functional connectivity. Both of these metrics were calculated within each individual landscape.
with x a gridcell containing GI at distance; n the number of gridcells containing GI; d from the target site (in km); and grid size 0.000625 ha (2.5 × 2.5 m), which, although constant, is retained to allow potential comparability with other studies. Landscapes consist of circles with a 1600-m radius from the centre of the target site.
To model connectivity, we assumed the green infrastructure was potential grassland habitat. The contribution of each GI element to connectivity was calculated and summed across all grid cells (n) containing GI (x) of each landscape using Eq. 1. Two versions were calculated: (1) structural connectivity, which used Euclidian distances to all GI habitats from the edge of focal grassland (d) and (2) inferred functional connectivity, whereby distance (d) was represented by the length of the least cost path between the edge of the focal grassland and a grid cell containing GI (x). We further refer to this least cost path as cost distance. For each grid cell containing GI, cost distance was calculated using the ArcGIS 10.6.1 spatial analyst Costs distance procedure (see Supplementary Material S1). Input into this procedure is a habitat-specific resistance layer from which the cost distance between the focal site and grid cell containing GI is computed. This resistance layer represents the reduced probability of dispersal of grassland plant seeds across higher resistance habitats by livestock as dispersal vectors, and follows the methodology in Adriaensen et al. (2003) and Sawyer et al. (2011).
For most road verges and semi-natural grasslands, there was no resistance to plant dispersal and establishment. For other landscape elements, the resistance was increased by 5-times for semi-permeable habitats (e.g. forest borders, hedgerows), larger for nearly-impassable habitats/land uses (forest, arable, urban: 100 times) and 1000-times for impregnable habitats/land uses (water, wetlands and railroads). We explain the full cost resistance procedure and all the resistance values employed in Supplementary Material S1. This was done for all 36 landscapes individually for both time periods, providing metrics of the levels of structural and functional connectivity in each landscape, for both the past and present-day.
We assessed changes in land use over time by classifying digitised land use in rasters of 5 metre spatial resolution and summing transitions or stasis from past to present for several key habitat types. This provided an indication of the contemporary status of areas that were dense forest (combined deciduous and coniferous), open forest, arable land, improved grassland or semi-natural grassland habitats in the past (Fig. 1). We also calculated total changes in the areas of major land use categories and green infrastructure habitats separately for each country (Fig. 2a), along with change in landscape structural and functional connectivity (Fig. 2b).
Plant species data
We recorded all vascular plant species present within ten 1 m2 quadrats, randomly located within each focal grassland patch (total 360 plots). This ensured that biodiversity sampling was independent of grassland patch size. We standardized species names across countries using the R package Taxonstand (Cayuela et al. 2019), and the International Plant Names Index nomenclature (IPNI 2020). We calculated total species richness for each focal grassland by summing the number of unique species found across all ten sampling plots, as a measure of the overall (gamma) diversity present within the grassland, and average smaller-scale species richness (alpha diversity) as the mean number of species found per plot. An additional full grassland survey was also carried out to identify all species present in the grassland. This added an average of 22.4 species per grassland on top of the plot totals. However, this full survey was not used in subsequent modelling, to avoid the risk of reducing comparability across countries due to different grassland sizes and greater potential observer bias. Results from this survey were used to confirm that plot totals across the ten plots were sufficient to represent grassland gamma diversity (Pearson correlation between plot total and full survey = 0.77, p < 0.001). We also calculated beta-diversity between plots in each focal grassland as the overall Sørensen dissimilarity between the ten survey plots using the beta.multi function in the R package betapart (Baselga et al. 2018). This allowed grasslands with species rich but patchy composition to be distinguished from grasslands with a highly diverse species composition across all plots. Gamma, alpha and beta diversity were also calculated for two subgroups of species (grassland specialist and generalist species) based on habitat preferences extracted from the TRY database (Kattge et al. 2011, 2020), trait ID 3096, containing two primary sources (Hill et al. 2004; Klimešová and Bello 2009). Grassland species were those favouring acid, calcareous, dry or neutral grassland broad habitat types, but not those capable of utilising arable or built-up habitats (Hill et al. 2004), nor those considered ruderal by Klimešová & de Bello (2009). Generalist species were those capable of utilising arable or built-up land uses (potentially in addition to other habitat types) along with those considered ruderal (Klimešová and Bello 2009). Although this is a coarse measure of habitat preference, taking a broad approach avoids problems with combining different expert classifications across multiple countries. For the 6.5% percent of occurrences with no data in TRY (including species only resolved to genus level), species were not assigned to either grassland specialist or generalist species (but are included in the overall category). See Supplementary Material S2 for a full list of species categories and a summary of their occurrence across countries. We used gamma, alpha and beta diversity for these three groups (overall, grassland and generalist species) as response variables in subsequent statistical analyses.
Statistical analyses
Statistical analysis consisted of two steps. First, since structural and functional connectivity are non-independent measures and cannot be included together in statistical models, we analysed their effects separately to determine which of these two variables had the most explanatory power for each biodiversity metric. In order to do this, we fitted two linear models for each response variable (9 response variables in total; alpha, beta and gamma diversity for all species, grassland specialists and generalist species) using landscape structural connectivity and functional connectivity, respectively, as single predictors. Comparing within these model pairs, the connectivity variable that produced the highest R-squared value for each response variable (Supplementary Material S3, Tables S3-1, S3-2 & S3-3) was then carried forward to a full model. This full model also included grassland patch area as a continuous predictor and history (restored or ancient) as a two-level factor, with ancient grasslands as the reference factor level. The potential importance of study country as a random effect was investigated by comparing a generalised least squares model without a random effect to a linear mixed effects (lme) model with country as a random intercept in a likelihood ratio test (Zuur et al. 2009). Only the models for patch total beta-diversity and grassland specialist beta-diversity were improved with the addition of country as a random effect. As a result, total beta-diversity and grassland specialist beta-diversity models were fit using the lme function in the R package nlme (Pinheiro et al. 2018), while all other models were fit as linear models using the lm function in R. Patch area and both structural and functional landscape connectivity were square root transformed to reduce the skew in their distribution. All variables in all models were centred and standardised by dividing by two standard deviations using the rescale function in R (Gelman 2008), to enable better comparison and selection between variables on different scales, and allow interpretation of coefficients in the presence of an interaction term (Schielzeth 2010). The interaction between landscape connectivity and patch history was also included, to account for any differences in the response of ancient and restored grasslands to varying connectivity. This resulted in nine final models of connectivity effects on gamma (Table 1), alpha (Table 2), and beta diversity (Table 3) in focal grassland patches. Model residuals were visually checked, confirming normality and homogeneity of distribution.
Results
Analysis of landscape change over the last 50 years demonstrated a consistent loss of semi-natural grassland across all three areas. This was driven mainly by the conversion of grasslands to forest, with a smaller proportion of grassland becoming arable land or improved grassland (Fig. 1). This figure also highlights the fact that many grasslands, particularly in Germany, have developed from former arable fields or from formerly forested land, with few grasslands with a long continuity of management remaining in the landscapes. There was also a complete loss of grazed semi-open forest habitat (which was not mapped in contemporary landscapes due to its absence) (Fig. 2a). Statistics from the resistance surfaces show that land use changes have had a large effect on landscape connectivity, with losses in both structural (mean decrease of 48.7%) and inferred functional connectivity (mean decrease of 33.4%) (Fig. 2b). Furthermore, almost all present-day landscapes have a lower connectivity than even the least well-connected landscapes in the past. This is despite the fact that linear green-infrastructure habitats became more frequent, particularly road verges and hedgerows, with the largest increases in these habitats occurring in the German landscapes (Fig. 2a).
Structural rather than functional connectivity was a more effective predictor of total gamma diversity within focal grasslands (Table 1). There was no significant relationship between connectivity and grassland specialist gamma diversity, but higher generalist gamma diversity was found where grassland structural connectivity was lower. Furthermore, the negative relationship between generalist species gamma diversity and structural connectivity was stronger in ancient patches than in restored, suggesting that a long continuity of grassland management in a highly connected landscape has an additional suppressive effect on the number of generalist species present. Although grassland specialist gamma diversity tended to be higher in ancient than restored grasslands, this relationship was not significant at the 95% confidence level. Lower generalist gamma diversity was seen with increasing grassland size, and in ancient patches compared to restored (Table 1).
Of the alpha diversity variables, only grassland specialist species were significantly affected by landscape connectivity (Table 2). Grassland specialist alpha diversity was most strongly affected by inferred functional connectivity, rather than structural connectivity. Management history was also important for grassland specialist alpha diversity, with ancient patches containing a higher alpha diversity of grassland specialist species per than restored grasslands. Beta diversity of all species between plots within the same grassland was lower in high structural connectivity landscapes, while beta-diversity of grassland specialists was lowest in high functional connectivity landscapes, and in restored patches (Table 3).
Discussion
Landscapes have undergone extensive losses of semi-natural grassland habitat over the last 50 years in our study areas across the three European countries, leading to substantial declines in both structural and functional connectivity for grassland plants. We show that observed increases in green-infrastructure habitats suitable for grassland plant species, such as hedgerows and road verges, are far from sufficient to compensate for the widespread abandonment of semi-natural grassland (Adriaens et al. 2006; Hooftman and Bullock 2012; Cousins et al. 2015). Although these habitats may contribute to functional connectivity (Vanneste et al. 2020), particularly at the local scale or in landscapes with very little remaining grassland, our results do not support the increasing focus on the potential of a well-connected network of green infrastructure to mitigate losses in core habitats at the landscape scale (Garmendia et al. 2016; Bullock et al. 2018). Consequently, landscapes today are less likely to facilitate grassland species dispersal, with no landscapes comparable in inferred functional connectivity to any but the least connected areas in the past. High connectivity is paramount to allow species to survive in fragmented landscapes, maintain vital plant/pollinator interactions and genetic diversity, and adapt or shift ranges in response to changes in climate and environmental conditions (Ozinga et al. 2009; Saura et al. 2014; Rotchés-Ribalta et al. 2018). The connectivity losses we observed, therefore, likely represent a serious decline in the ability of grassland plants to adapt to key current and future global change drivers.
Ancient grasslands are vital sources of biodiversity, since grassland specialist communities take many years to become fully established following the reintroduction of grazing management in restored sites (Aavik et al. 2008; Waldén et al. 2017; Karlík and Poschlod 2019). This was reflected here in differences observed between ancient and restored grasslands. Although total species richness was not affected by grassland age, ancient patches contained fewer generalist species, a higher alpha diversity of grassland species and a lower beta diversity between plots within each grassland compared to restored sites. This supports previous work suggesting that gamma diversity within grasslands increases relatively quickly following the re-introduction of traditional management measures, but that specialist species are slow to establish as larger populations (Austrheim and Olsson 1999; Pykälä et al. 2005; Aavik et al. 2008; Schmid et al. 2017). Importantly, our results indicate that this is not only determined by temporal processes and management continuity, but is also dependent on landscape connectivity. Mean grassland specialist alpha diversity was lower in patches embedded in poorly connected landscapes, even in older grasslands. This may be because in landscapes that have suffered from connectivity declines, many species may exist only in relatively small numbers as “sink” populations. Such species are likely to be at greater risk of future local extinction due to the disruption of important meta-population dynamics and the reduced likelihood of additional migration from combined neighbouring populations (Eriksson 1996; Evju et al. 2015). This creates multiple conservation problems. Firstly, connectivity declines are a direct threat to biodiversity in ancient grasslands, contributing to species extinctions (Plue and Cousins 2018). Secondly, connectivity declines reduce the capacity for grassland restoration. Ancient grasslands in low-connectivity landscapes are likely to be less able to act as an effective source of colonising individuals, effectively decreasing the size of the landscape species pool. The lower ability of these species to disperse across the landscape then further reduces the likelihood of species reaching target patches, representing a serious obstacle to efforts to restore landscape biodiversity (Baur 2014; Waldén et al. 2017). More active methods of restoration such as species translocation or the spreading of cut hay or hayseed from species rich grasslands, a common agricultural practice in historical times but now less frequently applied outside of conservation management (Poschlod and Bonn 1998), may represent a way of overcoming these functional connectivity declines. These methods are likely to assist the initial colonisation of restored sites by grassland specialist species. However, the extent to which these methods would be able to provide long-term benefits to high landscape functional connectivity and to an established historic grazing network, is unclear.
Grassland species alpha diversity responded more strongly to the functional connectivity metric than to structural connectivity. This suggests that physical connecting elements such as hedgerows and open forest borders are not providing significant additional functional connectivity for grassland species in these landscapes. This may partly be due to quality of these habitats for grassland plants relative to core semi-natural grassland patches, which was built into the functional connectivity metric but not included in the structural connectivity metric (Auffret and Cousins 2013). While marginal habitats can support a range of grassland species, this can depend on the presence of favourable local environmental conditions (Jakobsson et al. 2018; Lindgren et al. 2018). If this is the case, managing these habitats more effectively for grassland species may help to increase functional connectivity, particularly in landscapes which have very little grassland remaining. However, the stronger relationship of grassland species density with the functional connectivity metric may also represent the importance of moving livestock as dispersal vectors for these plants (Fischer et al. 1996; Römermann et al. 2008; Plue et al. 2019). Taking this into a wider landscape context, well-connected core semi-natural habitat, maintained by moving livestock, is an important priority to preserve.
The occurrence of generalist species in semi-natural grasslands was negatively affected by structural connectivity. This also appears to underpin a negative relationship between structural connectivity and overall species diversity. There are several possible explanations for this. Firstly, the higher dispersal capability of generalist species means that they are less affected by landscape configuration, allowing them to reach all suitable patches regardless of landscape configuration (Römermann et al. 2008; Saura and Rubio 2010). Furthermore, establishment is a key element of effective dispersal (Auffret et al. 2017). Hence, it may be that generalist species are able to utilise green infrastructure connecting elements but are unable to fully establish in core-semi-natural sites due to their grazing intolerance (Vandewalle et al. 2014). Since only focal grasslands were sampled in this study, generalist species may be more common in the wider landscape in highly connected landscapes. However, where these species are less able to survive in ancient grasslands, i.e. sites with a long-history of regular, low intensity local management and grassland species present at higher densities, the higher landscape connectivity provided by green infrastructure is likely to have no direct impact on the number of species present in focal grasslands. In fact, as appears to be the case here, the positive effect of connectivity on grassland species may lead to a reduction in the number of generalist species present due to competition with the greater density of grassland species (that are more able to tolerate low-intensity grazing and dry, infertile conditions). Finally, the connectivity metrics were derived with grassland species in mind, and as such may be less appropriate for generalist species. Including other landscape elements as potential habitat may have further clarified these patterns.
Although linear landscape elements can act as pathways for undesirable plants to spread (Lelong et al. 2007; Maheu-Giroux and de Blois 2007; Joly et al. 2011), the presence of such habitats within the landscape does not appear to lead to an increase in the occurrence of generalist species in grasslands in these study areas. Differences between patterns observed for different species groups also highlight the fact that important biodiversity responses to landscape functional connectivity may underlie patterns in overall species richness. The functional connectivity metric here primarily considered dispersal via moving livestock, since this is a primary mechanism of biodiversity maintenance in species rich grassland habitats (Plue and Cousins 2018), and because the introduction of rotational grazing is often a focus of habitat restoration and landscape management plans. However, dispersal via bird, wildlife and humans through agricultural and conservation management provide some degree of additional spatial dispersal potential for many species (e.g. Auffret and Cousins 2013), and may depend upon different spatial assumptions than those applied here regarding landscape resistance. Hence, while the functional connectivity metric used here was able to explain patterns in grassland specialist diversity, different spatial assumptions may result in connectivity estimates which vary in their ability to explain patterns across species groups, and species with different dispersal specialisations.
The landscape changes we identified likely represent only the most recent part of an ongoing loss of grassland habitat. The total reductions in grassland connectivity are likely to be far more extensive (Adriaens et al. 2006; Poschlod et al. 2008; Cousins et al. 2015). Given this long-term history of habitat loss in these study landscapes, it is possible that time-lags remain in the response of some grassland species to past habitat fragmentation (Lindborg and Eriksson 2004; Helm et al. 2006; Piqueray et al. 2011). Any remaining extinction debts still to be settled in areas which have lost habitat may mean that the full negative effects of connectivity loss have not yet become fully apparent, although this likely only applies to older grassland habitats (Helm et al. 2006; Cousins 2009). Potential time lags and small-scale variation in environmental conditions within grasslands (particularly restored sites) may well represent important additional predictors of species diversity which were not accounted for in models here (Gazol et al. 2012). Despite this, grassland species alpha and beta diversity was well explained by the combination of landscape connectivity and patch history. This shows the key importance of these variables for grassland biodiversity, highlighting the role landscape connectivity plays in both maintaining healthy older grasslands (Hooftman et al. 2015; Plue and Cousins 2018) and allowing grassland species to colonise recently created habitat (Pywell et al. 2002; Waldén et al. 2017). Protecting remaining functional connections, particularly between older grasslands, seems the key to maintain grassland biodiversity. Passive restoration efforts on former grassland sites are likely to meet with limited success unless restored sites are connected functionally to ancient grasslands either via adjacent habitat or the movement of grazing livestock.
References
Aavik T, Jõgar Ü, Liira J, Tulva I, Zobel M (2008) Plant diversity in a calcareous wooded meadow—the significance of management continuity. J Veg Sci 19:475–484
Adriaens D, Honnay O, Hermy M (2006) No evidence of a plant extinction debt in highly fragmented calcareous grasslands in Belgium. Biol Conserv 133:212–224
Adriaensen F, Chardon JP, De Blust G, Swinnen E, Villalba S, Gulinck H, Matthysen E (2003) The application of ‘least-cost’ modelling as a functional landscape model. Landsc Urban Plan 64:233–247
Aguilar R, Cristóbal-Pérez EJ, Balvino-Olvera FJ, Aguilar-Aguilar M, Aguirre-Acosta N, Ashworth L, Lobo JA, Martén-Rodríguez S, Fuchs EJ, Sanchez-Montoya G, Bernardello G, Quesada M (2019) Habitat fragmentation reduces plant progeny quality: a global synthesis. Ecol Lett. https://doi.org/10.1111/ele.13272
Auffret AG, Cousins SAO (2013) Grassland connectivity by motor vehicles and grazing livestock. Ecography 36:1150–1157
Auffret AG, Kimberley A, Plue J, Waldén E (2018) Super-regional land-use change and effects on the grassland specialist flora. Nat Commun 9:3464
Auffret AG, Plue J, Cousins SAO (2015) The spatial and temporal components of functional connectivity in fragmented landscapes. Ambio 44:51–59
Auffret AG, Rico Y, Bullock JM, Hooftman DAP, Pakeman RJ, Soons MB, Suárez-Esteban A, Traveset A, Wagner HH, Cousins SAO (2017) Plant functional connectivity—integrating landscape structure and effective dispersal. J Ecol 105:1648–1656
Austrheim G, Olsson EGA (1999) How does continuity in grassland management after ploughing affect plant community patterns? Plant Ecol 145:59–74
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F (2018) betapart: partitioning beta diversity into turnover and nestedness components. R package version 1.5.1. https://CRAN.R-project.org/package=betapart
Baur B (2014) Dispersal-limited species—a challenge for ecological restoration. Basic Appl Ecol 15:559–564
Bullock JM, Bonte D, Pufal G, da Silva Carvalho C, Chapman DS, García C, García D, Matthysen E, Mar Delgado M (2018) Human-mediated dispersal and the rewiring of spatial networks. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2018.09.008
Cayuela L, Macarro I, Stein A, Oksanen J (2019) Taxonstand: taxonomic standardization of plant species names. R package version 2.2. https://CRAN.Rproject.org/package=Taxonstand
Cousins SAO (2006) Plant species richness in midfield islets and road verges—the effect of landscape fragmentation. Biol Conserv 127:500–509
Cousins SAO (2009) Extinction debt in fragmented grasslands: paid or not? J Veg Sci 20:3–7
Cousins SAO, Auffret AG, Lindgren J, Tränk L (2015) Regional-scale land-cover change during the 20th century and its consequences for biodiversity. Ambio 44:17–27
Damschen EI, Brudvig LA, Burt MA, Fletcher RJ Jr, Haddad NM, Levey DJ, Orrock JL, Resasco J, Tewksbury JT (2019) Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Science 365:1478–1480
Eriksson O (1996) Regional dynamics of plants: a review of evidence for remnant, source-sink and metapopulations. Oikos 77:248
Eriksson O, Cousins SAO, Bruun HH (2002) Land-use history and fragmentation of traditionally managed grasslands in Scandinavia. J Veg Sci 13:743–748
Evju M, Blumentrath S, Skarpaas O, Stabbetorp OE, Sverdrup-Thygeson A (2015) Plant species occurrence in a fragmented grassland landscape: the importance of species traits. Biodivers Conserv 24:547–561
Fagan KC, Pywell RF, Bullock JM, Marrs RH (2008) Do restored calcareous grasslands on former arable fields resemble ancient targets? The effect of time, methods and environment on outcomes. J Appl Ecol 45:1293–1303
Fischer SF, Poschlod P, Beinlich B (1996) Experimental studies on the dispersal of plants and animals on sheep in calcareous grasslands. J Appl Ecol 33:1206
Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–40
Garmendia E, Apostolopoulou E, Adams WM, Bormpoudakis D (2016) Biodiversity and green infrastructure in Europe: boundary object or ecological trap? Land Use Policy 56:315–319
Gazol A, Tamme R, Takkis K, Kasari L, Saar L, Helm A, Pärtel M (2012) Landscape and small scale determinants of grassland species diversity: direct and indirect influences. Ecography 35:944–951
Gelman A (2008) Scaling regression inputs by dividing by two standard deviations. Stat Med 27:2865–2873
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song DX, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci Adv 1(2):e1500052
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63(1):151–162
Helm A, Hanski I, Pärtel M (2006) Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett 9:72–77
Hill MO, Preston CD, Roy DB (2004) PLANTATT—attributes of British and Irish plants: status, size, life history, geography and habitats. Centre for Ecology and Hydrology, Huntingdon
Hooftman DAP, Bullock JM (2012) Mapping to inform conservation: a case study of changes in semi-natural habitats and their connectivity over 70 years. Biol Conserv 145:30–38
Hooftman DAP, Edwards B, Bullock JM (2015) Reductions in connectivity and habitat quality drive local extinctions in a plant diversity hotspot. Ecography 38:001–010
Hunter ML Jr, Acuña V, Bauer DM, Bell KP, Calhoun AJK, Felipe-Lucia MR, Fitzsimons JA, González E, Kinnison M, Lindenmayer D, Lundquist CJ, Medellin RA, Nelson EJ, Poschlod P (2017) Conserving small natural features with large ecological roles: A synthetic overview. Biol Conserv 211:88–95
IPNI (2020) International Plant Names Index. Published on the Internet http://www.ipni.org, The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. (Retrieved 07 Aug 2020)
Jakobsson S, Bernes C, Bullock JM, Verheyen K, Lindborg R (2018) How does roadside vegetation management affect the diversity of vascular plants and invertebrates? A systematic review. Environ Evid 7:1–14
Jakobsson S, Fukamachi K, Cousins SAO (2016) Connectivity and management enables fast recovery of plant diversity in new linear grassland elements. J Veg Sci 27:19–28
Joly M, Bertrand P, Gbangou RY, White MC, Dubé J, Lavoie C (2011) Paving the way for invasive species: Road type and the spread of Common ragweed (Ambrosia artemisiifolia). Environ Manage 48:514–522
Karlík P, Poschlod P (2019) Identifying plant and environmental indicators of ancient and recent calcareous grasslands. Ecol Indic 104:405–421
Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, Van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin FS, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernández-Méndez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kühn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusià J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Müller SC, Nadrowski K, Naeem S, Niinemets Ü, Nöllert S, Nüske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordoñez J, Overbeck G, Ozinga WA, Patiño S, Paula S, Pausas JG, Peñuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negret B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana J-F, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) TRY—a global database of plant traits. Glob Chang Biol 17:2905–2935
Kattge J, Bönisch G, Díaz S, Lavorel S, Prentice IC, Leadley P, Tautenhahn S, Werner GDA, Aakala T, Abedi M, Acosta ATR, Adamidis GC, Adamson K, Aiba M, Albert CH, Alcántara JM, Alcázar C C, Aleixo I, Ali H, Amiaud B, Ammer C, Amoroso MM, Anand M, Anderson C, Anten N, Antos J, Apgaua DMG, Ashman TL, Asmara DH, Asner GP, Aspinwall M, Atkin O, Aubin I, Baastrup-Spohr L, Bahalkeh K, Bahn M, Baker T, Baker WJ, Bakker JP, Baldocchi D, Baltzer J, Banerjee A, Baranger A, Barlow J, Barneche DR, Baruch Z, Bastianelli D, Battles J, Bauerle W, Bauters M, Bazzato E, Beckmann M, Beeckman H, Beierkuhnlein C, Bekker R, Belfry G, Belluau M, Beloiu M, Benavides R, Benomar L, Berdugo-Lattke ML, Berenguer E, Bergamin R, Bergmann J, Bergmann Carlucci M, Berner L, Bernhardt-Römermann M, Bigler C, Bjorkman AD, Blackman C, Blanco C, Blonder B, Blumenthal D, Bocanegra-González KT, Boeckx P, Bohlman S, Böhning-Gaese K, Boisvert-Marsh L, Bond W, Bond-Lamberty B, Boom A, Boonman CCF, Bordin K, Boughton EH, Boukili V, Bowman DMJS, Bravo S, Brendel MR, Broadley MR, Brown KA, Bruelheide H, Brumnich F, Bruun HH, Bruy D, Buchanan SW, Bucher SF, Buchmann N, Buitenwerf R, Bunker DE, Bürger J, Burrascano S, Burslem DFRP, Butterfield BJ, Byun C, Marques M, Scalon MC, Caccianiga M, Cadotte M, Cailleret M, Camac J, Camarero JJ, Campany C, Campetella G, Campos JA, Cano-Arboleda L, Canullo R, Carbognani M, Carvalho F, Casanoves F, Castagneyrol B, Catford JA, Cavender-Bares J, Cerabolini BEL, Cervellini M, Chacón-Madrigal E, Chapin K, Chapin FS, Chelli S, Chen SC, Chen A, Cherubini P, Chianucci F, Choat B, Chung KS, Chytrý M, Ciccarelli D, Coll L, Collins CG, Conti L, Coomes D, Cornelissen JHC, Cornwell WK, Corona P, Coyea M, Craine J, Craven D, Cromsigt JPGM, Csecserits A, Cufar K, Cuntz M, da Silva AC, Dahlin KM, Dainese M, Dalke I, Dalle Fratte M, Dang-Le AT, Danihelka J, Dannoura M, Dawson S, de Beer AJ, De Frutos A, De Long JR, Dechant B, Delagrange S, Delpierre N, Derroire G, Dias AS, Diaz-Toribio MH, Dimitrakopoulos PG, Dobrowolski M, Doktor D, Dřevojan P, Dong N, Dransfield J, Dressler S, Duarte L, Ducouret E, Dullinger S, Durka W, Duursma R, Dymova O, E-Vojtkó A, Eckstein RL, Ejtehadi H, Elser J, Emilio T, Engemann K, Erfanian MB, Erfmeier A, Esquivel-Muelbert A, Esser G, Estiarte M, Domingues TF, Fagan WF, Fagúndez J, Falster DS, Fan Y, Fang J, Farris E, Fazlioglu F, Feng Y, Fernandez-Mendez F, Ferrara C, Ferreira J, Fidelis A, Finegan B, Firn J, Flowers TJ, Flynn DFB, Fontana V, Forey E, Forgiarini C, François L, Frangipani M, Frank D, Frenette-Dussault C, Freschet GT, Fry EL, Fyllas NM, Mazzochini GG, Gachet S, Gallagher R, Ganade G, Ganga F, García-Palacios P, Gargaglione V, Garnier E, Garrido JL, de Gasper AL, Gea-Izquierdo G, Gibson D, Gillison AN, Giroldo A, Glasenhardt MC, Gleason S, Gliesch M, Goldberg E, Göldel B, Gonzalez-Akre E, Gonzalez-Andujar JL, González-Melo A, González-Robles A, Graae BJ, Granda E, Graves S, Green WA, Gregor T, Gross N, Guerin GR, Günther A, Gutiérrez AG, Haddock L, Haines A, Hall J, Hambuckers A, Han W, Harrison SP, Hattingh W, Hawes JE, He T, He P, Heberling JM, Helm A, Hempel S, Hentschel J, Hérault B, Hereş AM, Herz K, Heuertz M, Hickler T, Hietz P, Higuchi P, Hipp AL, Hirons A, Hock M, Hogan JA, Holl K, Honnay O, Hornstein D, Hou E, Hough-Snee N, Hovstad KA, Ichie T, Igić B, Illa E, Isaac M, Ishihara M, Ivanov L, Ivanova L, Iversen CM, Izquierdo J, Jackson RB, Jackson B, Jactel H, Jagodzinski AM, Jandt U, Jansen S, Jenkins T, Jentsch A, Jespersen JRP, Jiang GF, Johansen JL, Johnson D, Jokela EJ, Joly CA, Jordan GJ, Joseph GS, Junaedi D, Junker RR, Justes E, Kabzems R, Kane J, Kaplan Z, Kattenborn T, Kavelenova L, Kearsley E, Kempel A, Kenzo T, Kerkhoff A, Khalil MI, Kinlock NL, Kissling WD, Kitajima K, Kitzberger T, Kjøller R, Klein T, Kleyer M, Klimešová J, Klipel J, Kloeppel B, Klotz S, Knops JMH, Kohyama T, Koike F, Kollmann J, Komac B, Komatsu K, König C, Kraft NJB, Kramer K, Kreft H, Kühn I, Kumarathunge D, Kuppler J, Kurokawa H, Kurosawa Y, Kuyah S, Laclau JP, Lafleur B, Lallai E, Lamb E, Lamprecht A, Larkin DJ, Laughlin D, Le Bagousse-Pinguet Y, le Maire G, le Roux PC, le Roux E, Lee T, Lens F, Lewis SL, Lhotsky B, Li Y, Li X, Lichstein JW, Liebergesell M, Lim JY, Lin YS, Linares JC, Liu C, Liu D, Liu U, Livingstone S, Llusià J, Lohbeck M, López-García Á, Lopez-Gonzalez G, Lososová Z, Louault F, Lukács BA, Lukeš P, Luo Y, Lussu M, Ma S, Maciel Rabelo Pereira C, Mack M, Maire V, Mäkelä A, Mäkinen H, Malhado ACM, Mallik A, Manning P, Manzoni S, Marchetti Z, Marchino L, Marcilio-Silva V, Marcon E, Marignani M, Markesteijn L, Martin A, Martínez-Garza C, Martínez-Vilalta J, Mašková T, Mason K, Mason N, Massad TJ, Masse J, Mayrose I, McCarthy J, McCormack ML, McCulloh K, McFadden IR, McGill BJ, McPartland MY, Medeiros JS, Medlyn B, Meerts P, Mehrabi Z, Meir P, Melo FPL, Mencuccini M, Meredieu C, Messier J, Mészáros I, Metsaranta J, Michaletz ST, Michelaki C, Migalina S, Milla R, Miller JED, Minden V, Ming R, Mokany K, Moles AT, Molnár A, Molofsky J, Molz M, Montgomery RA, Monty A, Moravcová L, Moreno-Martínez A, Moretti M, Mori AS, Mori S, Morris D, Morrison J, Mucina L, Mueller S, Muir CD, Müller SC, Munoz F, Myers-Smith IH, Myster RW, Nagano M, Naidu S, Narayanan A, Natesan B, Negoita L, Nelson AS, Neuschulz EL, Ni J, Niedrist G, Nieto J, Niinemets Ü, Nolan R, Nottebrock H, Nouvellon Y, Novakovskiy A; Nutrient Network, Nystuen KO, O'Grady A, O'Hara K, O'Reilly-Nugent A, Oakley S, Oberhuber W, Ohtsuka T, Oliveira R, Öllerer K, Olson ME, Onipchenko V, Onoda Y, Onstein RE, Ordonez JC, Osada N, Ostonen I, Ottaviani G, Otto S, Overbeck GE, Ozinga WA, Pahl AT, Paine CET, Pakeman RJ, Papageorgiou AC, Parfionova E, Pärtel M, Patacca M, Paula S, Paule J, Pauli H, Pausas JG, Peco B, Penuelas J, Perea A, Peri PL, Petisco-Souza AC, Petraglia A, Petritan AM, Phillips OL, Pierce S, Pillar VD, Pisek J, Pomogaybin A, Poorter H, Portsmuth A, Poschlod P, Potvin C, Pounds D, Powell AS, Power SA, Prinzing A, Puglielli G, Pyšek P, Raevel V, Rammig A, Ransijn J, Ray CA, Reich PB, Reichstein M, Reid DEB, Réjou-Méchain M, de Dios VR, Ribeiro S, Richardson S, Riibak K, Rillig MC, Riviera F, Robert EMR, Roberts S, Robroek B, Roddy A, Rodrigues AV, Rogers A, Rollinson E, Rolo V, Römermann C, Ronzhina D, Roscher C, Rosell JA, Rosenfield MF, Rossi C, Roy DB, Royer-Tardif S, Rüger N, Ruiz-Peinado R, Rumpf SB, Rusch GM, Ryo M, Sack L, Saldaña A, Salgado-Negret B, Salguero-Gomez R, Santa-Regina I, Santacruz-García AC, Santos J, Sardans J, Schamp B, Scherer-Lorenzen M, Schleuning M, Schmid B, Schmidt M, Schmitt S, Schneider JV, Schowanek SD, Schrader J, Schrodt F, Schuldt B, Schurr F, Selaya Garvizu G, Semchenko M, Seymour C, Sfair JC, Sharpe JM, Sheppard CS, Sheremetiev S, Shiodera S, Shipley B, Shovon TA, Siebenkäs A, Sierra C, Silva V, Silva M, Sitzia T, Sjöman H, Slot M, Smith NG, Sodhi D, Soltis P, Soltis D, Somers B, Sonnier G, Sørensen MV, Sosinski EE Jr, Soudzilovskaia NA, Souza AF, Spasojevic M, Sperandii MG, Stan AB, Stegen J, Steinbauer K, Stephan JG, Sterck F, Stojanovic DB, Strydom T, Suarez ML, Svenning JC, Svitková I, Svitok M, Svoboda M, Swaine E, Swenson N, Tabarelli M, Takagi K, Tappeiner U, Tarifa R, Tauugourdeau S, Tavsanoglu C, Te Beest M, Tedersoo L, Thiffault N, Thom D, Thomas E, Thompson K, Thornton PE, Thuiller W, Tichý L, Tissue D, Tjoelker MG, Tng DYP, Tobias J, Török P, Tarin T, Torres-Ruiz JM, Tóthmérész B, Treurnicht M, Trivellone V, Trolliet F, Trotsiuk V, Tsakalos JL, Tsiripidis I, Tysklind N, Umehara T, Usoltsev V, Vadeboncoeur M, Vaezi J, Valladares F, Vamosi J, van Bodegom PM, van Breugel M, Van Cleemput E, van de Weg M, van der Merwe S, van der Plas F, van der Sande MT, van Kleunen M, Van Meerbeek K, Vanderwel M, Vanselow KA, Vårhammar A, Varone L, Vasquez Valderrama MY, Vassilev K, Vellend M, Veneklaas EJ, Verbeeck H, Verheyen K, Vibrans A, Vieira I, Villacís J, Violle C, Vivek P, Wagner K, Waldram M, Waldron A, Walker AP, Waller M, Walther G, Wang H, Wang F, Wang W, Watkins H, Watkins J, Weber U, Weedon JT, Wei L, Weigelt P, Weiher E, Wells AW, Wellstein C, Wenk E, Westoby M, Westwood A, White PJ, Whitten M, Williams M, Winkler DE, Winter K, Womack C, Wright IJ, Wright SJ, Wright J, Pinho BX, Ximenes F, Yamada T, Yamaji K, Yanai R, Yankov N, Yguel B, Zanini KJ, Zanne AE, Zelený D, Zhao YP, Zheng J, Zheng J, Ziemińska K, Zirbel CR, Zizka G, Zo-Bi IC, Zotz G, Wirth C (2020) TRY plant trait database—enhanced coverage and open access. Glob Chang Biol 26:119–188
Klimešová J, Bello F (2009) CLO-PLA: the database of clonal and bud bank traits of Central European flora. J Veg Sci 20:511–516
Kuemmerle T, Levers C, Erb K, Estel S, Jepsen MR, Müller D, Plutzar C, Stürck J, Verkerk PJ, Verburg PH, Reenberg A (2016) Hotspots of land use change in Europe. Env Res Lett 11:064020
Lelong B, Lavoie C, Jodoin Y, Belzile F (2007) Expansion pathways of the exotic common reed (Phragmites australis): a historical and genetic analysis. Divers Distrib 13:430–437
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845
Lindgren J, Kimberley A, Cousins SAO (2018) The complexity of forest borders determines the understorey vegetation. Appl Veg Sci 21:85–93
Maheu-Giroux M, de Blois S (2007) Landscape ecology of Phragmites australis invasion in networks of linear wetlands. Landsc Ecol 22:285–301
McGuire JL, Lawler JJ, McRae BH, Nuñez A, Theobald DM (2016) Achieving climate connectivity in a fragmented landscape. Proc Natl Acad Sci USA 113:7195–7200
Newbold T, Hudson LN, Hill SL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Díaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison ML, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DL, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HR, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JP, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50
Ozinga WA, Römermann C, Bekker RM, Prinzing A, Tamis WLM, Schaminée JHJ, Hennekens SM, Thompson K, Poschlod P, Kleyer M, Bakker JP, van Grenendael JM (2009) Dispersal failure contributes to plant losses in NW Europe. Ecol Lett 12:66–74
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: linear and nonlinear mixed effects models. R package version 3.1-137. https://CRAN.Rproject.org/package=nlme
Piqueray J, Cristofoli S, Bisteau E, Palm R, Mahy G (2011) Testing coexistence of extinction debt and colonization credit in fragmented calcareous grasslands with complex historical dynamics. Landsc Ecol 26:823–836
Piqueray J, Ferroni L, Delescaille LM, Speranza M, Mahy G, Poschlod P (2015) Response of plant functional traits during the restoration of calcareous grasslands from forest stands. Ecol Indic 48:408–416
Plue J, Aavik T, Cousins SAO (2019) Grazing networks promote plant functional connectivity among isolated grassland communities. Divers Distrib 25:102–115
Plue J, Cousins SAO (2018) Seed dispersal in both space and time is necessary for plant diversity maintenance in fragmented landscapes. Oikos 127:780–791
Poschlod P, Bonn S (1998) Changing dispersal processes in the central European landscape since the last ice age—an explanation for the actual decrease of plant species richness in different habitats. Acta Bot Neerl 47:27–44
Poschlod P, Braun-Reichert R (2017) Small natural features with large ecological roles in ancient agricultural landscapes of Central Europe—history, value, status, and conservation. Biol Conserv 211:60–68
Poschlod P, Karlík P, Baumann A, Wiedmann B (2008) The history of dry calcareous grasslands near Kallmünz (Bavaria) reconstructed by the application of palaeoecological, historical and recent-ecological methods. Hum Nat Stud Hist Ecol Environ Hist Inst Bot Czech Acad Sci Brno, CZ 130–143
Poschlod P, Kiefer S, Tränkle U, Fischer S, Bonn S (1998) Plant species richness in calcareous grasslands as affected by dispersability in space and time. Appl Veg Sci 1:75–91
Pykälä J, Luoto M, Heikkinen RK, Kontula T (2005) Plant species richness and persistence of rare plants in abandoned semi-natural grasslands in northern Europe. Basic Appl Ecol 6:25–33
Pywell RF, Bullock JM, Hopkins A, Walker KJ, Sparks TH, Burke MJW, Peel S (2002) Restoration of species-rich grassland on arable land: assessing the limiting processes using a multi-site experiment. J Appl Ecol 39:294–309
Römermann C, Tackenberg O, Jackel AK, Poschlod P (2008) Eutrophication and fragmentation are related to species’ rate of decline but not to species rarity: Results from a functional approach. Biodivers Conserv 17:591–604
Rotchés-Ribalta R, Winsa M, Roberts SPM, Öckinger E (2018) Associations between plant and pollinator communities under grassland restoration respond mainly to landscape connectivity. J Appl Ecol 55:2822–2833
Saura S, Bodin Ö, Fortin MJ (2014) Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J Appl Ecol 51:171–182
Saura S, Rubio L (2010) A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 33:523–537
Sawyer SC, Epps CW, Brashares JS (2011) Placing linkages among fragmented habitats: do least-cost models reflect how animals use landscapes? J Appl Ecol 48:668–678
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schleicher A, Biedermann R, Kleyer M (2011) Dispersal traits determine plant response to habitat connectivity in an urban landscape. Landsc Ecol 26:529–540
Schmid BC, Poschlod P, Prentice HC (2017) The contribution of successional grasslands to the conservation of semi-natural grasslands species—a landscape perspective. Biol Conserv 206:112–119
Taylor PD, Fahrig L, Henein K, Merriam G (1993) Connectivity is a vital element of landscape structure. Oikos 68:571–573
Thiele J, Kellner S, Buchholz S, Schirmel J (2018) Connectivity or area: what drives plant species richness in habitat corridors? Landsc Ecol 33:173–181
Tischendorf L, Fahrig L (2000) On the usage and measurement of landscape connectivity. Oikos 90:7–19
van Dijk WF, Ruijven J, Van, Berendse F, Snoo GR, De (2014) The effectiveness of ditch banks as dispersal corridor for plants in agricultural landscapes depends on species’ dispersal traits. Biol Conserv 171:91–98
Vandewalle M, Purschke O, De Bello F, Prentice HC, Lavorel S, Johansson LJ, Sykes MT (2014) Functional responses of plant communities to management, landscape and historical factors in semi-natural grasslands. J Veg Sci 25:750–759
Vanneste T, Govaert S, De Kesel W, Van Den Berge S, Vanganbeke P, Meeussen C, Brunet J, Cousins SAO, Decocq G, Diekmann M, Graae BJ, Hedwall PO, Heinken T, Helsen K, Kapás RE, Lenoir J, Liira J, Lindmo S, Litza K, Naaf T, Orczewska A, Plue J, Wulf M, Verheyen K, De Frenne P (2020) Plant diversity in hedgerows and road verges across Europe. J App Ecology 57:1244–1257
Waldén E, Öckinger E, Winsa M, Lindborg R (2017) Effects of landscape composition, species pool and time on grassland specialists in restored semi-natural grasslands. Biol Conserv 214:176–183
WallisDeVries MF, Poschlod P, Willems JH (2002) Challenges for the conservation of calcareous grasslands in northwestern Europe: integrating the requirements of flora and fauna. Biol Conserv 104:265–273
Watson JEM, Jones KR, Fuller RA, Di Marco M, Segan DB, Butchart SHM, Allan JR, McDonald-Madden E, Venter O (2016) Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conserv Lett 9:413–421
Wilson JB, Peet RK, Dengler J, Pärtel M (2012) Plant species richness: the world records. J Veg Sci 23:796–802
Winsa M, Bommarco R, Lindborg R, Marini L, Öckinger E (2015) Recovery of plant diversity in restored semi-natural pastures depends on adjacent land use. Appl Veg Sci 18:413–422
Zuur AF, Leno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was funded through the 2015–2016 BiodivERsA COFUND call for research proposals, with the national funders the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), the Swedish Environmental Protection Agency (Naturvårdsverket), the Belgian Science Policy Office (BelSPo), the Germany Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung, FKZ: 01LC1619A) and the Spanish Ministry of Science, Innovation and Universities (Ministerio de Ciencia, Innovación y Universidades). The authors would also like to acknowledge the fieldwork carried out by Maria Björk, Kasper van Acker, Robbe Cool and Lotje Vanhove. Sabine Fischer supported us with air photograph material.
Funding
Open access funding provided by Stockholm University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimberley, A., Hooftman, D., Bullock, J.M. et al. Functional rather than structural connectivity explains grassland plant diversity patterns following landscape scale habitat loss. Landscape Ecol 36, 265–280 (2021). https://doi.org/10.1007/s10980-020-01138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-020-01138-x