Abstract
Context
By modifying ecosystems, land cover changes influence the emergence, the spread and the incidence of vector-borne diseases.
Objective
This study aimed at identifying associations between landscape structure and the prevalence of two tick-borne infectious agents, Anaplasma phagocytophilum and Borrelia burgdorferi s.l., in small mammal communities.
Methods
Small mammals were sampled in 24 sites along a gradient of woodland fragmentation and hedgerow network density, and screened for infectious agents with real-time PCR techniques. For each site, structural variables (composition and configuration) of the surrounding landscape at various scales (0–500 m) and variables of wooded habitats connectivity based on graph theory and least cost path distances for the two dominant species, bank voles (Myodes glareolus) and wood mice (Apodemus sylvaticus), were computed.
Results
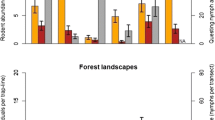
The A. phagocytophilum prevalence increased with wooded habitats cover (0–500 m), likely through host population size, and increased slightly with bank vole abundance, which has a higher reservoir competence than wood mouse. The B. burgdorferi s.l. prevalence increased with wooded ecotones only at local scales (50–100 m). Wooded habitats connectivity measures did not improve models built with simple land cover variables. A more marked spatial pattern was observed for the prevalence of A. phagocytophilum than B.burgdorferi s.l.
Conclusions
This study highlights the interest of considering together the ecology of infectious agents (e.g. host specificity) and the host species community ecology to better understand the influence of the landscape structure on the spatial distribution of vector-borne infectious agents.


Similar content being viewed by others
References
Agoulon A, Malandrin L, Lepigeon F, Vénisse M, Bonnet S, Becker CAM, Hoch T, Bastian S, Plantard O, Beaudeau F (2012) A vegetation index qualifying pasture edges is related to Ixodes ricinus density and to Babesia divergens seroprevalence in dairy cattle herds. Vet Parasitol 185:101–109
Al Hassan D, Georgelin E, Delattre T, Burel F, Plantegenest M, Kindlmann P, Butet A (2012) Does the presence of grassy strips and landscape grain affect the spatial distribution of aphids and their carabid predators? Agric For Entomol 15:24–33
Allan BF, Keesing F, Ostfeld RS (2003) Effect of forest fragmentation on Lyme disease risk. Conserv Biol 17:267–272
Araya-Anchetta A, Busch JD, Scoles GA, Wagner DM (2015) Thirty years of tick population genetics: a comprehensive review. Infect Genet Evol 29:164–179
Begon M, Hazel SM, Telfer S, Bown K, Carslake D, Cavanagh R, Chantrey J, Jones T, Bennett M (2003) Rodents, cowpox virus and islands: densities, numbers and thresholds. J Anim Ecol 72:343–355
Blaňarová L, Stanko M, Carpi G, Miklisová D, Víchová B, Mošanský L, Bona M, Derdáková M (2014) Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis 5:928–938
Boussard H, Baudry J (2014) Chloe2012: a software for landscape pattern analysis. https://www.rennes.inra.fr/sad/Outils-Produits/Outils-informatiques/Chloe
Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH (2003) Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis 9:63–70
Bown KJ, Lambin X, Ogden NH, Begon M, Telford G, Woldehiwet Z, Birtles RJ (2009) Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis 15:1948–1954
Bown KJ, Lambin X, Telford G, Heyder-Bruckner D, Ogden NH, Birtles RJ (2011) The common shrew (Sorex araneus): a neglected host of tick-borne infections? Vector-borne Zoonotic Dis 11:947–953
Bown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, Birtles RJ (2008) Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol 74:7118–7125
Boyard C, Vourc’h G, Barnouin J, (2008) The relationships between Ixodes ricinus and small mammal species at the woodland-pasture interface. Exp Appl Acarol 44:61–76
Cayol C, Jääskeläinen A, Koskela E, Kyröläinen S, Mappes T, Siukkola A, Kallio ER (2018) Sympatric Ixodes-tick species: pattern of distribution and pathogen transmission within wild rodent populations. Sci Rep 8:16660
Chastagner A, Moinet M, Perez G, Roy E, Mccoy KD, Plantard O, Agoulon A, Bastian S, Butet A, Rantier Y, Verheyden H, Cèbe N, Leblond A, Vourc’h G (2016) Prevalence of Anaplasma phagocytophilum in small rodents in France. Ticks Tick Borne Dis 7:988–991
Clay CA, Lehmer EM, Jeor SS, Dearing MD (2009) Testing mechanisms of the dilution effect: deer mice encounter rates, sin nombre virus prevalence and species diversity. Ecohealth 6:250–259
Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C (1997) Recommendations for euthanasia of experimental animals: part 2. Lab Anim 31:1–32
Coipan EC, Sprong H (2016) Ecology of Borrelia burgdorferi sensu lato. In: Braks MAH, van Wieren SE, Takken W, Sprong H (eds) Ecology and prevention of Lyme borreliosis. Wageningen Academic Publishers, Wageningen, pp 41–61
Courtney JW, Kostelnik LM, Zeidner NS, Massung RF (2004) Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol 42:3164–3168
Eskildsen A (2010) Effects of resource abundance on habitat selection and spatial behavior of the bank vole (Myodes glareolus). Master’s Thesis, University of Copenhagen
Ferrante M, González E, Lövei GL (2017) Predators do not spill over from forest fragments to maize fields in a landscape mosaic in central Argentina. Ecol Evol 7:1–9
Foltête JC, Clauzel C, Vuidel G (2012) A software tool dedicated to the modelling of landscape networks. Environ Model Softw 38:316–327
Gassner F, Verbaarschot P, Smallegange RC, Spitzen J, Van Wieren SE, Takken W (2008) Variations in Ixodes ricinus density and Borrelia infections associated with cattle introduced into a woodland in The Netherlands. Appl Environ Microbiol 74:7138–7144
Gehring TM, Swihart RK (2003) Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: mammalian predators in an agricultural landscape. Biol Conserv 109:283–295
Gern L, Estrada-Peña A, Frandsen F, Gray JS, Jaenson TG, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall PA (1998) European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl Bakteriol 287:196–204
Gern L, Siegenthaler M, Hu CM, Leuba-Garcia S, Humair PF, Moret J (1994) Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): Duration and enhancement of infectivity for Ixodes ricinus ticks. Eur J Epidemiol 10:75–80
Gil-Tena A, Nabucet J, Mony C, Abadie J, Saura S (2014) Woodland bird response to landscape connectivity in an agriculture-dominated landscape: a functional community approach. Community Ecol 15:256–268
Gottdenker NL, Chaves LF, Calzada JE, Saldaña A, Carroll CR (2012) Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. PLoS Negl Trop Dis 6:e1884
Guivier E, Galan M, Chaval Y, Xuéreb A, Ribas Salvador A, Poulle M-L, Voutilainen L, Henttonen H, Charbonnel N, Cosson JF (2011) Landscape genetics highlights the role of bank vole metapopulation dynamics in the epidemiology of Puumala hantavirus. Mol Ecol 20:3569–3583
Halos L, Bord S, Cotté V, Gasqui P, Abrial D, Barnouin J, Boulouis H-J, Vayssier-Taussat M, Vourc’h G (2010) Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl Environ Microbiol 76:4413–4420
Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F (2012) Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis 18:1951–1957
Heylen D, Tijsse E, Fonville M, Matthysen E, Sprong H (2013) Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ Microbiol 15:663–673
Hoch T, Monnet Y, Agoulon A (2010) Influence of host migration between woodland and pasture on the population dynamics of the tick Ixodes ricinus: a modelling approach. Ecol Modell 221:1798–1806
Hofmeester TR, Coipan EC, Van Wieren SE, Prins HHT, Takken W, Sprong H (2016) Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ Res Lett 11:043001
Hofmeester TR, Jansen PA, Wijnen HJ, Coipan EC, Fonville M, Prins HHT, Sprong H, van Wieren SE (2017) Cascading effects of predator activity on tick-borne disease risk. Proc R Soc B Biol Sci 284:20170453
Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT (2013) Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8:e54341
Hubbard MJ, Baker AS, Cann KJ (1998) Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th century, assessed by PCR. Med Vet Entomol 12:89–97
Humair PF, Rais O, Gern L (1999) Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33–42
Humair PF, Turrian N, Aeschlimann A, Gern L (1993) Borrelia burgdorferi in a focus of Lyme borreliosis: epizootiologic contribution of small mammals. Folia Parasitol 40:65–70
Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76:297–307
Jahfari S, Coipan EC, Fonville M, Docters van Leeuwen A, Hengeveld P, Heylen D, Heyman P, van Maanen C, Butler CM, Földvári G, Szekeres S, Van Duijvendijk G, Tack W, Rijks JM, Van der Giessen J, Takken W, Van Wieren SE, Takumi K, Sprong H (2014) Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors 7:365
Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD (2013) Biodiversity decreases disease through predictable changes in host community competence. Nature 494:230–233
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993
Kallio ER, Begon M, Birtles RJ, Bown KJ, Koskela E, Mappes T, Watts PC (2014) First Report of Anaplasma phagocytophilum and Babesia microti in Rodents in Finland. Vector-borne Zoonotic Dis 14:389–393
Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H, Ostfeld RS (2009) Hosts as ecological traps for the vector of Lyme disease. Proc R Soc B Biol Sci 276:3911–3919
Kikkawa T (1964) Movement, activity and distribution of the small rodents Clethrionomys glareolus and Apodemus sylvaticus in Woodland. J Anim Ecol 33:259–299
Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell H-S, Brade V, Kraiczy P (2002a) Host association of Borrelia burgdorferi sensu lato: the key role of host complement. Trends Microbiol 10:74–79
Kurtenbach K, De Michelis S, Sewell HS, Etti S, Schafer SM, Holmes E, Hails R, Collares-Pereira M, Santos-Reis M, Hanincova K, Labuda M, Bormane A, Donaghy M (2002b) The key roles of selection and migration in the ecology of Lyme borreliosis. Int J Med Microbiol 291:152–154
Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH (2006) Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4:660–669
Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V (2010) Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr 9:54
Li S, Hartemink N, Speybroeck N, Vanwambeke SO (2012) Consequences of landscape fragmentation on Lyme disease risk: a cellular automata approach. PLoS ONE 7:e39612
Mader H (1984) Animal habitat isolation by roads and agricultural fields. Biol Conserv 29:81–96
Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T (2009) Tick-borne encephalitis virus: a review of an emerging zoonosis. J Gen Virol 90:1781–1794
Margos G, Vollmer SA, Cornet M, Garnier M, Fingerle V, Wilske B, Bormane A, Vitorino L, Collares-Pereira M, Drancourt M, Kurtenbach K (2009) A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol 75:5410–5416
Marsot M, Chapuis JL, Gasqui P, Dozières A, Masséglia S, Pisanu B, Ferquel E, Vourc’h G (2013) Introduced Siberian chipmunks (Tamias sibiricus barberi) contribute more to Lyme borreliosis risk than native reservoir rodents. PLoS ONE 8:1–8
Meunier FD, Corbin J, Verheyden C, Jouventin P (1999) Effects of landscape type and extensive management on use of motorway roadsides by small mammals. Can J Zool 77:108–117
Michel N, Burel F, Legendre P, Butet A (2007) Role of habitat and landscape in structuring small mammal assemblages in hedgerow networks of contrasted farming landscapes in Brittany, France. Landsc Ecol 22:1241–1253
Millán de la Peña N, Butet A, Delettre Y, Paillat G, Morant P, Le Du L, Burel F (2003) Response of the small mammal community to changes in western French agricultural landscapes. Landsc Ecol 18:265–278
Mortelliti A, Amori G, Boitani L (2010) The role of habitat quality in fragmented landscapes: a conceptual overview and prospectus for future research. Oecologia 163:535–547
Nupp TE, Swihart RK (1998) Effects of forest fragmentation on populations attributes of white-footed mice and eastern chipmunks. J Mammal 79:1234–1243
Nupp TE, Swihart RK (2000) Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. J Mammal 81:512–526
Ostfeld RS, Keesing F (2000) Biodiversity and disease risk: the case of Lyme disease. Conserv Biol 14:722–728
Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F (2014) Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS ONE 9:e107387
Ostfeld RS, LoGiudice K (2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84:1421–1427
Ouin A, Paillat G, Butet A, Burel F (2000) Spatial dynamics of wood mouse (Apodemus sylvaticus) in an agricultural landscape under intensive use in the Mont Saint Michel Bay (France). Agric Ecosyst Environ 78:159–165
Paillat G, Butet A (1996) Spatial dynamics of the bank vole (Clethrionomys glareolus) in a fragmented landscape. Acta Ecol 17:553–559
Papillon Y, Buffiere L, Butet A (2002) Rhodamine B as collective marker for small mammals. Acta Theriol (Warsz) 47:491–497
Perez G, Bastian S, Agoulon A, Bouju A, Durand A, Faille F, Lebert I, Rantier Y, Plantard O, Butet A (2016) Effect of landscape features on the relationship between Ixodes ricinus ticks and their small mammal hosts. Parasite Vectors 9:1–18
Perez G, Bastian S, Chastagner A, Agoulon A, Plantard O, Vourc’h G, Butet A (2017) Ecological factors influencing small mammal infection by Anaplasma phagocytophilum and Borrelia burgdorferi s.l. in agricultural and forest landscapes. Environ Microbiol 19:4205–4219
Pisanu B, Chapuis JL, Dozières A, Basset F, Poux V, Vourc’h G (2014) High prevalence of Borrelia burgdorferi s.l. in the European red squirrel Sciurus vulgaris in France. Ticks Tick Borne Dis 5:1–6
R Development Core Team (2019) R v.3.6.1. R foundation for statistical computing
Randolph SE (1975a) Seasonal dynamics of a host-parasite system: Ixodes trianguliceps (Acarina: Ixodidae) and its small mammal hosts. J Anim Ecol 44:425–449
Randolph SE (1975b) Patterns of distribution of the tick Ixodes trianguliceps birula on its hosts. J Anim Ecol 44:451–474
Reidsma P, Tekelenburg T, vand den Berg M, Alkemade R (2006) Impacts of land-use change on biodiversity: An assessment of agricultural biodiversity in the European Union. Agric Ecosyst Environ 114:86–102
Rico A, Kindlmann P, Sedláček F (2007) Barrier effects of roads on movements of small mammals. Folia Zool 56:1–12
Rizzoli A, Hauffe HC, Carpi G, Vourc’h GI, Neteler M, Rosà R (2011) Lyme borreliosis in Europe. Eurosurveillance 16:1–8
Roche B, Rohani P, Dobson AP, Guégan J-F (2013) The impact of community organization on vector-borne pathogens. Am Nat 181:1–11
Rollend L, Fish D, Childs JE (2013) Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis 4:46–51
Rosso F, Tagliapietra V, Baráková I, Derdáková M, Konečný A, Hauffe HC, Rizzoli A, Kone A, Hauffe HC, Rizzoli A (2017) Prevalence and genetic variability of Anaplasma phagocytophilum in wild rodents from the Italian alps. Parasite Vectors 10:293
Rubio A, Ávila-Flores R, Suzán G (2014) Responses of small mammals to habitat fragmentation: epidemiological considerations for rodent-borne hantaviruses in the Americas. EcoHealth 11:526–533
Ruiz-Capillas P, Mata C, Malo JE (2015) How many rodents die on the road? Biological and methodological implications from a small mammals’ roadkill assessment on a Spanish motorway. Ecol Res 30:417–427
Saura S, Pascual-Hortal L (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landsc Urban Plan 83:91–103
Sinski E, Pawelczyk A, Bajer A, Behnke JM (2006) Abundance of wild rodents, ticks and environmental risk of Lyme borreliosis: a longitudinal study in an area of Mazury Lakes district of Poland. Ann Agric Environ Med 13:295–300
Skuballa J, Petney T, Pfäffle M, Oehme R, Hartelt K, Fingerle V, Kimmig P, Taraschewski H (2012) Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis 3:8–13
Stuen S, Granquist EG, Silaghi C (2013) Anaplasma phagocytophilum: a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 4:1–33
Suzán G, García-Peña GE, Castro-Arellano I, Rico O, Rubio AV, Tolsá MJ, Roche B, Hosseini PR, Rizzoli A, Murray KA, Zambrana-Torrelio C, Vittecoq M, Bailly X, Aguirre AA, Daszak P, Prieur-Richard A-H, Mills JN, Guégan J-F (2015) Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecol Evol 5:865–873
Szacki J (1987) Ecological corridor as a factor determining the structure and organization of a bank vole population. Acta Theriol (Warsz) 32:31–44
Szacki J, Babińska-werka J, Liro A (1993) The influence of landscape spatial structure on small mammal movements. Acta Theriol (Warsz) 38:113–123
Szekeres S, Coipan EC, Rigó K, Majoros G, Jahfari S, Sprong H, Földvári G (2015) Eco-epidemiology of Borrelia miyamotoi and Lyme borreliosis spirochetes in a popular hunting and recreational forest area in Hungary. Parasite Vectors 8:1–8
Tattersall FH, Macdonald DW, Hart BJ, Johnson P, Manley W, Feber R (2002) Is habitat linearity important for small mammal communities on farmland? J Appl Ecol 39:643–652
Tew TE, Macdonald DW (1994) Dynamics of space use and male vigour amongst wood mice, Apodemus sylvaticus, in the cereal ecosystem. Behav Ecol Sociobiol 34:337–345
van Apeldoorn RC, Oostenbrink WT, van Winden A, van der Zee FF (1992) Effects of fragmentation on the bank vole, Clethrionomys glareolus, in an agricultural landscape. Oikos 65:265–274
Vourc’h G, Boyard C, Barnouin J (2008) Mammal and bird species distribution at the woodland-pasture interface in relation to the circulation of ticks and pathogens. Ann N Y Acad Sci 1149:322–325
Zhang Z, Usher MB (1991) Dispersal of wood mice and bank voles in an agricultural landscape. Acta Theriol (Warsz) 36:239–245
Acknowledgements
We are very grateful to Agnès Bouju, Floriane Boullot, Axelle Durand, Mathieu Gonnet, Olivier Jambon, Maggy Jouglin, Emmanuelle Moreau, Pranav Pandit, and Ionut Pavel who helped in sampling and preparing small mammal tissues before molecular analyses; to Séverine Barry, Amélie Cohadon, Angélique Pion, and Valérie Poux who helped in the lab for the molecular detection of infectious agents; and to Nelly Dorr and Isabelle Lebert who managed the data base of the OSCAR project (https://www6.inra.fr/oscar/). We thank the ‘Zone Atelier Armorique’ (https://osur.univ-rennes1.fr/za-armorique/) for providing the GIS data and for the access to its field facilities. We thank Henri Lemercier and Benoît Chevallier from the ‘Office National des Forêts’ for facilitating the access to the Villecartier forest. The ‘Tiques et Maladies à Tiques’ team of the ‘Réseau Ecologie des Interactions Durables’ group, supported by the INRA and the CNRS gave a rich thinking environment. This work was funded by the French National Research Agency (ANR-11-Agro-001-04; call for Proposal ‘Agrobiosphere’, OSCAR project). This work is part of the PhD of GP, which was supported by a fellowship from the Brittany region, France. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
GP, SB, AA, GV, OP and AB designed the study. GP, SB, AA, YR, OP and AB participated to the small mammal field sampling. AC performed most of the DNA extractions and the molecular analyses. YR managed the GIS data. GP performed all data analyses and drafted the manuscript. All authors read, commented and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10980_2019_957_MOESM2_ESM.ods
Supplementary file2 (ODS 27 kb). Appendix 1: Example of the habitats landscape connectivity analyses. Final aggregated land cover 5 m-resolution raster file (a) and ‘dPC’ (‘difference in Probability of Connectivity’) metric computed on different graphs (b, c, and d). The sampling patch on the right (surrounded in blue) appears moderately connected with Euclidian distances (b), while it is weakly connected when weighted by least cost paths (c and d) because it is surrounded by a river at North and by roads on other sides (see a). The sampling patch on the left is moderately connected for the wood mouse (c) while it is weakly connected for the bank vole (d) because it is separated to other wooded habitat patches by large grassland or crops patches resulting in fewer connections for this latter species. See Materials and Methods for more details.
Rights and permissions
About this article
Cite this article
Perez, G., Bastian, S., Chastagner, A. et al. Relationships between landscape structure and the prevalence of two tick-borne infectious agents, Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato, in small mammal communities. Landscape Ecol 35, 435–451 (2020). https://doi.org/10.1007/s10980-019-00957-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00957-x