Abstract
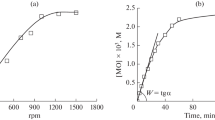
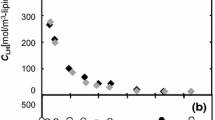
The kinetics of low-temperature autooxidation of methyl oleate for a bimolecular mechanism of the degenerate branched reaction were analyzed taking into account changes in the methyl oleate concentration for different amounts of peroxides formed. This analysis made it possible to explain the experimental data. The role of the initial peroxide concentration in the mechanism and kinetics of the chain degenerate branched reaction of methyl oleate autooxidation was studied in the steady-state approximation and in the course of establishing a steady-state concentration of radicals. A systematic approach to estimating the antioxidant properties of lipids on the basis of the methyl oleate model was proposed.
Similar content being viewed by others
REFERENCES
N.M. Emanuel’ E.T. Denisov Z.K. Maizus (1965) Tsepnye reaktsii okisleniya uglevodorodov v zhidkoi faze Nauka Moscow
N.M. Emanuel’ Yu.N. Lyaskovskaya (1961) Tormozhenie protsessov okisleniya zhirov Pishchepromizdat Moscow
N.M. Emanuel G.E. Zaikov Z.K. Maizus (1984) Oxidation of Organic Compounds. Effect of Medium Pergamon Oxford
Yu.A. Vladimirov A.I. Archakov (1972) Perekisnoe okislenie lipidov v biologicheskikh membranakh Nauka Moscow
E.B. Burlakova N.G. Khrapova (1985) Usp. Khim. 54 IssueID9 1540
E.B. Burlakova A.V. Alesenko E.M. Molochkina N.P. Pal’mina N.G. Khrapova (1975) Bioantioksidanty v luchevom porazhenii i zlokachestvennom roste Nauka Moscow
E.N. Frankel (1980) Prog. Lipid Res. 19 1
E.N. Frankel (1987) Chem. Phys. Lipid 44 IssueID2–4 73
L.N. Shishkina (1992) Issledovanie sinteticheskikh i prirodnykh antioksidantov in vitro i in vivo Nauka Moscow 26
Shishkina, L.N., Doctoral (Chem.) Dissertation, Moscow, 2003.
N. Yanishlieva I. Skibida Z. Maizus A. Popov (1971) Izv. Bolg. Akad. Nauk, Khim. Otd. 4 1
Shishkina, L.N., Men’shov, V.A., and Brin, E.F., Izv. Akad. Nauk, Ser. Biol., 1996, no. 3, p. 292.
T.D. Nekipelova A.B. Gagarina (1982) Neftekhimiya 22 IssueID2 278
Gagarina, A.B., Nekipelova, T.D., and Emanuel’, N.M., Khim. Fiz., 1983, no. 12, p. 1666.
A.B. Gagarina T.D. Nekipelova N.M. Emanuel’ (1982) Dokl. Akad. Nauk SSSR 264 IssueID6 1412
E.B. Burlakova N.M. Storozhok N.G. Khrapova V.V. Naumov E.N. Kukhtina (1990) Vopr. Med. Khim. 36 IssueID4 72
L.N. Shishkina N.V. Polyakova Yu.P. Taran (1994) Rad. Biol. Radioekologiya 34 IssueID3 362
Burlakova, E.B., Mazaletskaya, L.I., Sheludchenko, N.I., and Shishkina, L.N., Izv. Akad. Nauk, Ser. Khim., 1995, no. 6, p. 1053.
Author information
Authors and Affiliations
Additional information
Translated from Kinetika i Kataliz, Vol. 45, No. 6, 2004, pp. 848–858.
Original Russian Text Copyright © 2004 by Khrustova, Shishkina.
Rights and permissions
About this article
Cite this article
Khrustova, N.V., Shishkina, L.N. The role of peroxides in the mechanism of low-temperature autooxidation of methyl oleate and its solutions with lipids. Kinet Catal 45, 799–808 (2004). https://doi.org/10.1007/s10975-005-0038-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10975-005-0038-3