Abstract
Gestrinone is an active pharmaceutical ingredient used in the treatment of endometriosis as capsules, with ongoing evaluation for intravaginal administration, while also having been studied for its potential antitumoral effects. The purpose of this study was to determine the compatibility of gestrinone with four excipients used in the development of solid pharmaceutical formulations (α-lactose monohydrate, magnesium stearate, starch, and talc) and to obtain a fully characterized thermoanalytical profile of gestrinone with the help of kinetic analysis. Preformulation studies were carried out on 1:1 mass/mass binary mixtures between gestrinone and each excipient by instrumental screening under ambient conditions using ATR-FTIR spectroscopy investigations, and later by studying the effect of thermal treatment over the samples (TG/DTG/DSC). The obtained results suggest that under ambient conditions, no chemical interactions take place between the active pharmaceutical ingredient and selected excipients, whereas under thermal stress incompatibilities are observed in all systems. The mechanism of decomposition was preliminary evaluated by the ASTM E698 and later completed by the isoconversional methods of Friedman, Kissinger–Akahira–Sunose, and Flynn–Wall–Ozawa, which suggest similar mean activation energies. The mechanism of decomposition was elucidated in the last part of the study, by employing the modified NPK method. This method suggests that gestrinone is thermally degraded by the contribution of two individual processes, both consisting of superimposed physical transformations and chemical degradations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a chronic pelvic inflammatory disease that affects 5–10% of the feminine population of reproductive age. The clinical symptoms include pelvic pain, dysmenorrhea, non-menstrual pelvic pain, and infertility, being observed in approximately 50% of cases of women diagnosed with infertility. The first therapeutic approach is represented by pharmacological therapy, with the molecules used being: gonadotropin-releasing hormone agonist (leuprorelin), gonadotropin-releasing hormone antagonist (cetrorelix), progesterone, and gestrinone. In 2014, the European Society of Human Reproduction and Embryology issued a guideline with recommendations regarding the diagnosis and treatment of endometriosis, where gestrinone is the only anti-progestagen recommended for this pathology [1,2,3,4,5].
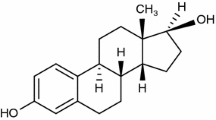
GTN, also known as ethyl-norgestrienone, chemical structure presented in Fig. 1, is a derivative of 19-nortestosterone which posses androgenic, weak estrogen-like, anti-estrogen, weak progestin, and strong progesterone effects. Gestrinone is an active pharmaceutical ingredient used in the treatment of endometriosis as capsules (sold under the name Dimetrose® and Nemestran®), with ongoing evaluation for intravaginal administration [6, 7], and recently investigated for its possible antitumor effect [8]. The molecular formula of GTN is C21H24O2, and its molecular weight is 308.4 g mol−1. It presents a low water solubility, namely, 0.00969 mg mL−1 [9, 10].
Owing to its therapeutical properties, the two confirmed and with ungoing investigations, a good biodisponibility, and limited number of pharmaceutical forms, it is imperative an evaluation of the thermoanalytical profile of the API under non-isothermal conditions and an establishment of its compatibility with pharmaceutical excipients, in order to preserve the chemical structure of the API during the pharmaceutical processing.
Even if there are several papers dealing with the biopharmaceutical profile (including pharmacokinetics) [11] and biological activity of GTN [1,2,3,4,5], no references were found in the literature in our area of interest, namely, thermal stability and preformulation studies.
According to this, we focused into investigating the compatibility of GTN with four widely used pharmaceutical excipients, namely: Lact·H2O, MgS, St, and T. To evaluate the compatibility of the API with the excipients, BMs were obtained and then analyzed with the following analytical methods: thermal investigations (TG, DTG, and DSC analysis) and ATR-FTIR investigations.
Considering the lack of information regarding the thermoanalytical profile of GTN, our second objective was to characterize the degradation mechanism of the API using kinetic analysis. To achieve this, a preliminary method was used, namely, ASTM E698, followed by two integral isoconversional methods (Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose), one differential isoconversional method (Friedman), and later by employing the modified NPK method.
The importance of kinetic studies is reflected in the fact that the biopharmaceutical profile is directly influenced by the chemical and physical transformations that occur during the preformulation stage of pharmaceutical formulations. In the case of hormonal therapy, where the doses used are very low (2.5 mg for GTN), the preservation of structural integrity of the API is crucial to ensure an optimal therapeutic response [12]. The decomposition of GTN or its interaction with the pharmaceutical excipients determines, in addition to a decrease in the concentration of the API in the pharmaceutical formulation, a therapeutic failure that will require re-evaluation of the patient and the search for new therapeutic solutions, which will cause additional stress on the medical system, considering that about 1–2 in 20 of women of reproductive age suffer from endometriosis [4].
The ASTM E698 method is a model-free non-isoconversional method, where a single activation energy is estimated without considering the reaction progress, with respect to single-step reactions. This kinetic method is described by the following equation [13, 14]:
By using the integral kinetic method Flynn–Wall–Ozawa, the changes in the apparent activation energy can be determined using distinct linear thermogravimetric curves, making this method more suited to a complex decomposition process. It uses the Doyle linear approximation for \(p\left(x\right)\), and it is defined by the equation (where g(α) is a constant value at a given value of α) [13, 15]:
The value of Ea is determined from the slope of the chart with the abscissa of \(\frac{1}{T}\) and the ordinate of lnβ, at a specific α [15].
Kissinger–Akahira–Sunose is an integral isoconversional kinetic method, where the apparent activation energy is determined from the slopes of the linear thermogravimetric curves. Each curve represents the \(\text{ln}\frac{\beta }{{T}^{2}}=f\left(\frac{1}{T}\right)\) at a constant value of the conversion degree. It uses the Murray and White approximation for \(p\left(x\right)\), and it is defined by the following equation (where g(α) is a constant value at a given value of α) [16, 17]:
The Friedman method is a differential isoconversional method and the most accurate one, as a result of a differential method for the processed data. The advantages of this kinetic method are that it allows the calculation of the activation energy without any approximations and that it can be used for an isothermal, non-isothermal, or complex heating processes. In order to obtain the values of Ea, the calculations should be performed at a constant α value, by using the equation [18]:
The mechanism of decomposition consisting of the kinetic triplet can be achieved by employing the non-parametric kinetic method, proposed initially by Serra, Sempere, and Nomen [19,20,21,22] and later modified by Vlase, Vlase, and Doca [23, 24]. The advantage of this method resides in the fact that it can produce the kinetic triplet without using an a priori model for k(T), nor for f(α), being based solely on the universal-accepted axiom that the reaction rate (r) can be expressed as a product of two separable functions—one dependent solely on temperature and one dependent solely on conversion:
Materials and methods
Preparation of the samples
Gestrinone was acquired from Santa Cruz Biotechnology, batch number: #K2811. The sample was used as received and kept under the conditions recommended by the supplier.
In order to establish whether there are any possible interactions between GTN and the pharmaceutical excipients, four binary physical mixtures were prepared with a mass ratio of 1:1 (w:w), in an agate mortar and triturated for 5 min. The obtained mixture was sealed in a brown vial and kept at − 20 °C, in the absence of light. The excipients used were: α-lactose monohydrate (from Sigma, Germany, batch no.: #SLBK4809V), magnesium stearate (from Sigma, Germany, batch no.: #SZBF2590V), starch (from Grain Processing Corporation, USA, batch no.: SZBF167), and talc (from Sigma, Germany, batch no.: #BCBQ7164V).
ATR-FTIR investigations
The spectra were acquired with the help of the Shimadzu Fourier Transform Infrared Spectrophotometer IR Tracer 100 instrument, which presents an attenuated total reflectance accessory. The wavenumber range was between 4000 and 400 cm−1, and the spectra were registered subsequential 20 consecutive measures at 4 cm−1.
Thermal investigations
To obtain the thermoanalytical data (TG—thermogravimetric/mass curve and DTG—derivative thermogravimetric/mass derivative), Setline TGA (SETARAM, Caluire, France) instrument and open alumina crucibles were used. The analysis was performed in dynamic air flow (100 mL min−1) under non-isothermal conditions, with five heating rates of β = 2, 4, 6, 8, and 10 °C min−1 and at one heating rate of β = 10 °C min−1 for the binary mixtures, from 40 up to 600 °C. The DSC curves for all the samples were recorded with a NETZSCH DSC 204F1 Phoenix (NETZSCH, Selb, Germany) instrument using aluminum crucibles in inert nitrogen flow (20 mL min−1) from 40 up to 250 °C.
Kinetic analysis
The kinetic analysis was performed for the first decomposition process of gestrinone using the software from AKTS AG TechnoArk, Siders, Switzerland, namely, Thermokinetics software version 4.46. The abbreviations and the protocol used respect all the recommendations of the International Confederation for Thermal Analysis and Calorimetry (ICTAC) Kinetics Committee. The fundamentals of the modified NPK method and its use were based on references from the literature [23].
Results and discussion
ATR-FTIR investigations
The data obtained from the spectral investigations employed for GTN, the BMs, and pure excipients are presented in Table 1, alongside Fig. 2, where the obtained ATR-FTIR spectra are presented. The wavenumbers written in bold for the BM signify the new absorption bands observed compared with those of the API and the corresponding excipient.
If a chemical interaction is present in the BM, on the ATR-FTIR spectrum it should be observed: either the shifting or the absence of some absorption bands characteristic for the two components or the appearance of new ones. [25, 26]. The spectra were interpreted according to the information given by the scientific literature [27, 28].
The spectrum of GTN shows an intense absorption band at 1653 cm−1, which characterizes the stretching vibrations of the carbonyl moiety. Usually, this absorption band appears at ≈1700 cm−1, but in the case of GTN, a slight shift can be observed, due to the conjugation with the double bonds determining the delocalization of the π electrons. Additionally, in the 1230–1100 cm−1 region, four medium-intensity bands can be noticed at: 1212; 1189; 1122; and 1106 cm−1, which describe the stretching vibrations of the C–C–C group and the bending of C–C(=O)–C in the C–C–C group. For the hydroxyl moiety, the spectra present a large absorption band with maxim at 3414 cm−1 characteristics for strong intermolecular hydrogen bonding. The stretching vibrations of the C–O bond determine the presence of an intense absorption peak at 1066 cm−1. The in-plane bending vibrations of the hydroxyl group are shown at 1423 and 1331 cm−1, which are usually coupled with the wagging vibrations of C–H moiety, determining a small diagnostic value for these peaks. The structure of GTN presents a monosubstituted terminal alkyne radical, for which the absorption in the spectral range 2140–2100 cm−1 is absent, probably due to its low intensity. To confirm the presence of the alkyne radical, a strong absorption band is observed at 3253 cm−1 caused by the stretching vibrations of the C–H bond, and the bending vibrations of the same bond can be noticed as a weak broad band with maxim at 1348 cm−1, which are over toned by the strong absorption peaks at 695, 673, and 635 cm−1. For the C=C bond, present in the cycloalkenes, its stretching vibrations are observed at 1661 cm−1, and the same vibrations for the C–H moiety are noticed at 3299 cm−1. In the case of methyl groups, the stretching vibrations are present at 2959 cm−1 (symmetric) and 2870 cm−1 (asymmetric), while the bending vibrations are observed at 1378 cm−1 (symmetric) and at 1449 cm−1 (asymmetric), respectively. Frequently, those asymmetrical bending vibrations posses an hindering effect on the scissoring of the methylene group; but due to the conjugation observed on the chemical structure of GTN, on the spectra, these vibrations are observed at 1437 cm−1. The stretching vibrations of the methylene and methylidyne radicals are noticed in the spectral range of 3100–2990 cm−1.
The ATR-FTIR spectrum of the mixture formed between GTN and Lact·H2O, at first glance, no significant changes can be observed. However, the absorption band of GTN from 3414 cm−1 is hindered by a wide absorption band determined by the hydroxyl moiety from the lactose and from its water molecule. The band at 1414 cm−1 noticed on the spectra of GTN can be observed as a shoulder for the absorption band in the 1447–1404 cm−1 range on the ATR-FTIR spectra of the BM. There are only two peaks of the API missing on the ATR-FTIR spectra of the BM, namely, 1122 and 1043 cm−1. For the excipient, all the absorption bands can be observed on the spectra of the BM. Taking into account the aforementioned observations, it can be concluded that at room temperature, there is no chemical interaction between GTN and Lact·H2O.
In the case of the binary mixture formed between GTN and St, the ATR-FTIR spectrum does not show any significant changes in the wavenumbers of the absorption bands of the API and the excipient, demonstrating the absence of a chemical interaction in this BM.
Regarding the spectra of the BM between GTN and MgS, the appearance of a new absorption band at 1013 cm−1 can be noticed, being the result of the merging of the band at 1011 cm−1 of GTN and 1018 cm−1 of MgS. The wide band in the 3517–3026 cm−1 region of with maximum at 3263 cm−1 observed on the ATR-FTIR spectra of MgS induces an intensification of the spectral region, characteristic to the stretching vibrations of the hydrogen intermolecular bonds, on the BM spectrum. Additionally, when the BM spectrum is compared to the one of the pure excipients, the missing of two absorption peaks can be observed at 1117 and 1076 cm−1. On the ATR-FTIR spectra of MgS, the carboxylate anion determines two absorption bands, the first one being a strong one with maximum at 1572 cm−1 due to the asymmetrical stretching vibrations and the second one with a maximal at 1464 cm−1 due to the symmetrical stretching vibrations. The second one, on the spectra of the BM, overlaps with the absorption band at 1437 cm−1 of GTN, and the peak observed at 1423 cm−1 for the API can be noticed as a shoulder for the same intense absorption band of the carboxylate anion. These results demonstrate the absence of a chemical interaction between the two compounds at ambient temperature.
When the spectrum of the BM of GTN + T was studied, no significant changes were observed at the wavenumber corresponding to the main absorption bands of the API and the excipient. Thus, being shown the lack of any chemical interactions in this BM at room temperature.
Thermal investigations
The results of the TG, DTG, and DSC analysis employed for GTN, the BMs, and pure excipients are presented in Table 2, alongside Figs. 3 and 4 attributed to the TG, DTG, and DSC curves obtained. The purpose of the thermal investigations at a heating rate of 10 °C min−1 was to observe if there are any interactions between the API and the excipient when heated. In order to confirm an interaction between the two compounds on the DSC curve, it should be noticed that in the case of a physical interaction, a reduction in the intensity of each peak of the API and the excipient and, in the case of a chemical interaction, a shifting or lack of the analytical signal characteristic to the API and the excipient or the appearance of new peaks. Considering the fact that the BM contains a mass ratio of 1:1 (w:w), the mass loss observed on the TG and DTG curves for each component of the BM should be reduced by 50% compared to one of the API and the pure excipients [26, 29, 30].
By analyzing the TG and DTG curves, a thermal stability for GTN up to 178 °C is showed, the high thermal stability being explained by the fact that gestrinone is a derivative of 19-nortestosterone. Also, the thermoanalytical curves revealed a multi-step decomposition process for GTN, consisting of three degradation steps. The first process noticed on the DSC curve is an endothermal solid-state transition (endothermic peak at 113 °C), and the second, the melting of GTN, is represented as an endothermic peak at 148 °C—the value being in agreement with the data reported in the literature (150–152 °C), the enthalpy being 47.65 J g−1 [9].
The thermoanalytical curves reveal a decomposition process consisting of three steps for Lact·H2O, the first one being the result of the loss of the water molecule from the excipient. The dehydration is observed as an endothermic process on the DSC curve with a maximum at 147 °C. Also, on the same curve, it can be noticed two more endothermic peaks at 220 and 239 °C. For the BM formed with GTN, the decomposition process consists in three-step degradation. The first one is associated with the dehydration of the excipient, rendered by the loss of half of the mass of the water lost by the pure excipient. In the case of the second process, it can be related with the first GTN degradation process and the second and third processes of Lact·H2O. For this one, the expected mass loss is approximately 39%, but the TG curve revealed a loss of mass equal to 33.10%. The fact that this process of the BM begins at 192 °C suggests the appearance of a chemical interaction between GTN and Lact·H2O under thermal stress. The third degradation step of the BM is completely different from the second and third degradation processes of GTN and excipient, confirming the fact that in the previous stage, chemical interactions took place. On the DSC curve of the physical mixture, it can be noticed the appearance of several new endothermic peaks, at 56, 129, 172, 209, and 219 °C, respectively, and one exothermic process at 166 °C, with the disappearance of the melting point of GTN. These changes confirm the existence of chemical interactions between the two compounds in the BM when heated.
Regarding St, the degradation process consists of two stages, the initial process being represented by dehydration with a Δm around 10% and associated with an endothermic peak at 105 °C on the DSC curve. The degradation of anhydrous St begins at 228 °C and ends at 346 °C with a Δm = 59.12%. For the BM, the thermoanalytical curves revealed a complex degradation process consisting in three processes for which the loss of mass is equal to the one expected, the first process representing the dehydration of the excipient. The DSC curve of the mixture presents one endothermic peak at 68 °C (associated with the dehydration, shifted to lower temperature because of mixing the excipient with API), while the melting point of GTN is no longer observed at 148 °C. All these aspects support the conclusion that GTN strongly interacts with St under thermal stress, so this excipient should be avoided in solid dosage forms.
The dehydration of MgS takes place in the thermal range of 68–117 °C, as revealed by the TG and DTG curves. On the DSC curve of the excipient, it can be noticed that this process happens in multiple stages, shown as two endothermic peaks at 91 and 116 °C. Also, on the same curve, an exothermic peak is presented at 165 °C. The other two processes revealed by the TG and DTG curves describe the degradation of MgS. On the other hand, by studying the TG and DTG curves of the BM, the degradation process consists of six stages. The DSC curve of the mixture shows a new endothermic peak at 127 °C, respectively, along with the reduced value of the enthalpy of fusion of the peak characteristic to GTN melting and the disappearance of the exothermic peak of the pure excipient. Considering these results, it can be concluded that between GTN and MgS exist a chemical interaction when heated.
In the case of T, the TG, DTG, and DSC curves do not show any significant process, since it is well known that this excipient has great thermal stability. For the BM, the TG and DTG show a two-step decomposition process. The first one can be correlated with the primary process noticed on the DTG curve of GTN, the first aspect observed is the reduction of the temperature at which this process begins, namely, 157 °C, suggesting that T determines a decrease in the thermal stability of GTN. On the DSC curve, it can be noticed a new endothermic peak at 56 °C, along with a shifting to a lower temperature (143 °C) of the melting point of the API, and a value of ΔHfus being less than expected. These results suggest the modification of thermal stability of GTN under the presence of T.
Kinetic analysis
For the kinetic investigations to be performed, data obtained from the first degradation process observed on the thermoanalytical curves of the API were used. The DTG curves were drawn up at five heating rates, namely, β = 2, 4, 6, 8, and 10 °C min−1, the selected temperature ranges being presented in Table 3.
The kinetic study was carried out according to ICTAC recommendations [31,32,33,34,35], namely, the use of isoconversional methods, two integral and one differential [36, 37] for obtaining the apparent Ea value, and later by employing the modified NPK method, for the determination of the kinetic triplet.
The analysis of reaction progress vs. temperature (Fig. 5a) reveals the consequence of the selected heating rates over the conversion, namely, the shift of it to higher temperatures, caused by the changes in the heat and mass transfer, which is affected by thermal inertia while the heating rates increase. Also, in the analysis of reaction rate dependence of temperature (Fig. 5b), the same tendency is noticed. However, since the aspect of the reaction rate curves suffers modifications with β, a complex pattern of decomposition is suspected.
The kinetic study was initially performed by an initial method, namely, ASTM E698 method. The plotting of the ASTM E698 method suggests an apparent activation energy around 99.1 kJ mol−1 (Fig. 6a), but this value is obtained by assuming a single-step mechanism for decomposition process of GTN, so an in-depth study must be carried out in order to confirm/infirm this supposition.
By employing the isoconversional methods of FR (Fig. 6b), FWO (Fig. 6c), and KAS (Fig. 6d), a family of lines were obtained, and an estimative value of the apparent activation energy (Ea) for each selected α was calculated, and the values are tabulated in Table 4.
The variation of the individual values of Ea versus α around the mean value is presented in Fig. 7a, b, and c. As can be observed, the FR method reveals a relative constancy of Ea values when α is less than 0.40, and with the increase in conversion, a decrease in Ea values is observed up to a minimum value at α = 0.85. At the end of the process, at α > 0.85, a significant increase in Ea is observed, out of the confidence range of Ēa ± 0.1·Ēa. The fact that a mechanism change occurs at α > 0.75 is also supported by the corroboration of these data with the tendencies observed in Fig. 5a and b, namely, the reaction progress depending on temperature and reaction rate depending on temperature. The FWO method suggests a mechanism that does not change with the heating rate, since the individual values of Ea versus α around the mean value are all placed within the confidence interval around the mean. However, a similar profile of variation is observed at higher conversion, for α > 0.80, as in the case of FR method. Furthermore, by employing the KAS method, each value of Ea falls in the interval Ēa ± 0.1·Ēa. Also, the same tendency of a sudden increase in the values of the apparent activation energy for α > 0.85 is noticed. The differences observed between the mean values according to FR method (95.5 ± 2.5 kJ mol−1), FWO method (104.4 ± 0.7 kJ mol−1), and KAS method (100.7 ± 0.8 kJ mol−1) are solely influenced by different processing of data (differential versus integral).
Since the isoconversional methods, both differential and integral, cannot provide the kinetic triplet, a more complex method must be employed, and we focused on the modified non-parametric kinetic method, i.e., NPK.
The NPK method presents the major advantage that it does not use any approximations, in comparison with the other isoconversional methods. The reaction rate is the result of two independent functions, namely: f(α) and k(T).
The nature of the physical or chemical processes that take place during the decomposition process are shown in Table 5, where n and m are the reaction orders suggested by the truncated universal-accepted kinetic model of Šesták and Berggren [38, 39]:
Using the mathematical formulas presented in the scientific literature, in Table 5, the results of the modified NPK method are tabulated, and in Fig. 8, a three-dimensional graph of the transformation rate surface is presented, the coordinates being β·dα/dT; α; T.
The explained variance λ describes the contribution of each process to the obtained mean value of the activation energy. In the case of GTN, two parallel processes can be observed contributing to the degradation, the value of the mean active energy being the result of the following equation:
The first GTN degradation process consists of two steps, which take place simultaneously, the first one being the major one, fact rendered by the value of λ1 = 86.1%, consists of both physical (m = 1/2) and chemical (n = 1) transformations. The value of the explained variance of the second process is significantly smaller than one for the first process, suggesting that its contribution to the main degradation is minor. The transformations that occur during the second process are a physical transformation (m = 1) and a chemical degradation (n = 1), respectively.
Conclusions
In this study, we have described the fully characterized thermoanalytical and kinetic profiles for a derivative of 19-nortestosterone, namely, gestrinone, and determined whether there is any chemical interaction of the hormone with four, the most used, pharmaceutical excipients, namely, α-lactose monohydrate, magnesium stearate, starch, and talc. The necessity of this study is due to the fact that no information was found in specialized literature regarding the stability profile of this API, and as well the lack of preformulation studies.
The compatibility with the selected excipients was carried out on binary mixtures containing the active pharmaceutical ingredient and the excipient in a mass ratio of 1:1, the instrumental methods used are ATR-FTIR and thermal analysis (TG, DTG, and DSC). The results showed the lack of chemical interactions between the components of the binary mixtures at ambient temperature, but were induced when the samples were subjected to thermal stress under non-isothermal conditions, in all cases.
The thermoanalytical investigations indicate a good stability of gestrinone up to 178 °C, associated with the presence of the 19-nortestosterone skeleton. The decomposition process of gestrinone consists of three degradation steps, and the DSC curve revealed an endothermal solid-state transition at 113 °C, and an endothermic peak at 148 °C, characteristic to solid–liquid phase transition of gestrinone.
For the kinetic studies, the first degradation process of gestrinone was investigated. The results of the three isoconversional kinetic methods used showed a complex decomposition process, with a mechanism change at α≈0.80, outcome also confirmed by the reaction progress depending on temperature and the reaction rate depending on temperature. For a more detailed evaluation of the mechanism underlying the degradation of gestrinone, the modified non-parametric kinetic method was applied: The results showed that the first decomposition of the active pharmaceutical ingredient consists of two individual processes, both being the result of physical transformations and chemical degradations.
Abbreviations
- GTN:
-
Gestrinone
- Lact·H2O:
-
α-Lactose monohydrate
- MgS:
-
Magnesium stearate
- St:
-
Starch
- T:
-
Talc
- API:
-
Active pharmaceutical ingredient
- BM:
-
Binary mixture
- FWO:
-
Flynn–Wall–Ozawa
- KAS:
-
Kissinger–Akahira–Sunose
- FR:
-
Friedman
- NPK:
-
Modified non-parametric kinetic method
- α :
-
Conversion degree
- t:
-
Time
- β :
-
Linear heating rate (C min−1)
- A :
-
The preexponential factor according to the kinetic model of Arrhenius (min–1)
- k(T):
-
The temperature function
- f(α):
-
The differential conversion function
- g(α):
-
The integral conversion function
- E a :
-
Activation energy (kJ mol–1)
- R :
-
Universal gas constant (J mol–1 K–1)
- T :
-
Absolute temperature (K)
- Δm :
-
Mass loss in a certain temperature interval
References
Dunselman GAJ, Vermeulen N, Becker C, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
de Souza Pinto LP, Ferrari G, dos Santos IK, de Mello Roesler CR, de Mello GI. Evaluation of safety and effectiveness of gestrinone in the treatment of endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2023;307(1):21–37.
Sachedina A, Todd N. Dysmenorrhea, endometriosis and chronic pelvic pain in adolescents. JCRPE J Clin Res Pediatr Endocrinol. 2020;12:7–17.
Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–52.
Johnson NP, Hummelshoj L, Adamson GD, et al. World endometriosis society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24.
Maia H, Saback W, Coutinho EM. Short term effects of the vaginal administration of gestrinone and Miodesin™ on endometriosis pain. J Clin Rev Case Rep. 2019;4(8):4–9.
Coutinho EM, Azadian-Boulanger G. Treatment of endometriosis by vaginal administration of gestrinone. Fertil Steril. 1988;49(3):418–22.
Ciou H-H, Lee T-H, Wang H-C, Ding Y-R, Tseng C-J, Wang P-H, Tsai M-H, Tzeng S-L. Repurposing gestrinone for tumor suppressor through P21 reduction regulated by JNK in gynecological cancer. Transl Res. 2022;243:21–32.
PubChem. Gestrinone. https://pubchem.ncbi.nlm.nih.gov/compound/16320-04-0. Accessed 5 Oct 2023
DrugBank. Gestrinone. https://go.drugbank.com/drugs/DB11619. Accessed 18 Mar 2024
Wang QG, Wu ZP, Wang YM, Luo GA, Wu E, Gao XF. Determination of gestrinone in human serum by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B. 2000;746(2):151–9.
Ali F, Kumar R, Sahu PL, Singh GN. Physicochemical characterization and compatibility study of roflumilast with various pharmaceutical excipients. J Therm Anal Calorim. 2017;130(3):1627–41.
Osman AI, Fawzy S, Farrell C, Al-Muhtaseb AH, Harrison J, Al-Mawali S, Rooney DW. Comprehensive thermokinetic modelling and predictions of cellulose decomposition in isothermal, non-isothermal, and stepwise heating modes. J Anal Appl Pyrolysis. 2022;161: 105427.
Fawzy S, Osman AI, Farrell C, Al-Muhtaseb AH, Harrison J, Al-Fatesh AS, Fakeeha AH, Doran J, Yang H, Rooney DW. Characterization and kinetic modeling for pyrolytic conversion of cotton stalks. Energy Sci Eng. 2021;9(10):1908–18.
Khawam A, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Nonisothermal kinetic studies. Thermochim Acta. 2005;436(1–2):101–12.
Segal E, Budrugeac P, Carp O, Doca N, Popescu C, Vlase T. Analiza termica: fundamente si aplicatii. Analiza cinetica a transformarilor heterogene. Editura Academiei Române, Bucureşti
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57(4):217.
Sbirrazzuoli N. Interpretation and physical meaning of kinetic parameters obtained from isoconversional kinetic analysis of polymers. Polymers (Basel). 2020. https://doi.org/10.3390/POLYM12061280.
Serra R, Sempere J, Nomen R. A new method for the kinetic study of thermoanalytical data: the non-parametric kinetics method. Thermochim Acta. 1998;316(1):37–45.
Serra R, Sempere J, Nomen R. The non-parametric kinetics. A new method for the kinetic study of thermoanalytical data. J Therm Anal. 1998;52(1):933–43.
Brown ME, Maciejewski M, Vyazovkin S, et al. Computational aspects of kinetic analysis part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000. https://doi.org/10.1016/S0040-6031(00)00443-3.
Ferrer N, Serra E, Nomen R, Sempere J. Thermal decomposition of tricyclohexylidene triperoxide. J Therm Anal Calorim. 2018;134(2):1293–8.
Vlase T, Vlase G, Doca N, Bolcu C. Processing of non-isothermal TG data: Comparative kinetic analysis with NPK method. J Therm Anal Calorim. 2005;80:59–64.
Segal E, Doca N, Budrugeac P, Popescu C, Carp O, Vlase T. Analiza termică. Fundamente și aplicații - Analiza cinetică a transformărilor eterogene. Editura Academiei Române, București
Segall AI. Preformulation: the use of FTIR in compatibility studies. J Innov Appl Pharm Sci. 2019;4(3):01–6.
Ali F, Nandi U, Trivedi M, Prakash A, Dahiya M, Sahu PL, Kumar R, Singh GN. Quantitative characterization and pharmaceutical compatibility between teneligliptin and widely used excipients by using thermal and liquid chromatography tandem mass spectrometry techniques. J Therm Anal Calorim. 2018;132(1):385–96.
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric identification of organic compounds. 8th ed. New York: Wiley; 2014.
Stuart BH. Infrared spectroscopy: fundamentals and applications. New Jersey: John Wiley & Sons; 2004.
Chadha R, Bhandari S. Drug-excipient compatibility screening-Role of thermoanalytical and spectroscopic techniques. J Pharm Biomed Anal. 2014;87:82–97.
Veras KS, Fachel FNS, Pittol V, Garcia KR, Bassani VL, dos Santos V, Henriques AT, Teixeira HF, Koester LS. Compatibility study of rosmarinic acid with excipients used in pharmaceutical solid dosage forms using thermal and non-thermal techniques. Saudi Pharm J. 2019;27(8):1138–45.
Brown ME, Maciejewski M, Vyazovkin S, et al. Computational aspects of kinetic analysis Part A: The ICTAC Kinetics Project-data, methods and results. Thermochim Acta. 2000;355(1–2):125–43.
Vyazovkin S. Computational aspects of kinetic analysis. Part C. The ICTAC kinetics project the—light at the end of the tunnel. Thermochim Acta. 2000. https://doi.org/10.1016/S0040-6031(00)00445-7.
Burnham AK. Computational aspects of kinetic analysis Part D: the ICTAC kinetics project—multi-thermal-history model-fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000. https://doi.org/10.1016/S0040-6031(00)00446-9.
Brown ME, Maciejewski M, Vyazovkin S, et al. Computational aspects of kinetic analysis. Thermochim Acta. 2000;355(1–2):125–43.
Verma RK, Szilagyi IM, Pielichowska K, Raftopoulos KN, Šimon P, Melnikov AP, Ivanov DA. Good laboratory practice in thermal analysis and calorimetry. J Therm Anal Calorim. 2023;148(6):2211–31.
Rodante F, Vecchio S, Tomassetti M. Kinetic analysis of thermal decomposition for penicillin sodium salts—model-fitting and model-free methods. J Pharm Biomed Anal. 2002;29:1031–43.
Duce C, Vecchio Ciprioti S, Ghezzi L, Ierardi V, Tinè MR. Thermal behavior study of pristine and modified halloysite nanotubes: a modern kinetic study. J Therm Anal Calorim. 2015;121(3):1011–9.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3(1):1–12.
Simon P. Fourty years of the Sestak-Berggren equation. Thermochim Acta. 2011. https://doi.org/10.1016/j.tca.2011.03.030.
Author information
Authors and Affiliations
Contributions
Amalia Ridichie, Cosmina Bengescu, Adriana Ledeți, and Gabriela Vlase helped in conceptualization; Amalia Ridichie, Cosmina Bengescu, Gerlinde Rusu, Răzvan Bertici, and Matilda Rădulescu helped in data curation; Amalia Ridichie, Titus Vlase, and Ionuţ Ledeţi helped in formal analysis; Adriana Ledeți, Titus Vlase, and Ionuţ Ledeţi helped in methodology; Matilda Rdulescu and Gabriela Vlase helped in resources; Titus Vlase and Gabriela Vlase worked in software; Gabriela Vlase helped in validation; and Amalia Ridichie, Ionuţ Ledeţi, Francisc Peter, and Adriana Ledeți contributed to writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ridichie, A., Bengescu, C., Ledeţi, A. et al. Thermal stability, preformulation, and kinetic degradation studies for gestrinone. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13520-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13520-x