Abstract
Levonorgestrel, also called the morning-after pill, is a synthetic progestogen presenting structural similarities with progesterone. It is mostly used for its contraceptive effect in the form of intrauterine devices, subcutaneous implants, patches, dragées, tablets, coated tablets and orodispersible tablets being the first contraceptive that was used containing only a derivative of progestogen. Because of its wide use and various pharmaceutical formulations, it is necessary to evaluate the presence of any potential interactions between the active pharmaceutical ingredient and pharmaceutical excipients in the development of new formulations which possess increased stability and shelf life. The purpose of this study was to assess if there are any interactions between levonorgestrel and several excipients used in the pharmaceutical field, having different roles in the development of solid formulations. The studies were carried out on binary mixtures containing levonorgestrel and excipient. For the formation of the binary mixture, each excipient was used in a 1:1 mass ratio with the substance of pharmaceutical interest, levonorgestrel and the instrumental investigation results were compared to the ones of the pure active pharmaceutical ingredient and excipients. Alongside thermal analysis (TG/DTG/DSC), ATR-FTIR spectroscopy and PXRD analysis were used to evaluate the occurrence of possible interactions. For a more accurate interpretation of the obtained results, statistical calculations were performed, namely obtaining the value of the correlation coefficient. The results show the lack of a chemical interaction between levonorgestrel and microcrystalline cellulose, sodium carboxymethyl cellulose, calcium lactate pentahydrate, α-lactose monohydrate and talc. In the case of the physical mixtures which contain as excipients: aspartame and magnesium stearate a chemical interaction is observed at ambient temperature and when heated. Regarding the binary mixtures which contain mannitol, starch and colloidal silicon dioxide at ambient temperature no chemical interaction is observed, but when heated it can be noticed the existence of a chemical interaction between levonorgestrel and those excipients. As concerns the BM between LNG and PVP, precautions should be taken into consideration, due to the possibility of a chemical interaction even at ambient temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
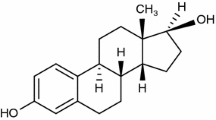
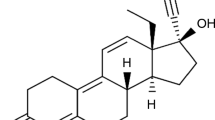
Levonorgestrel, also called the morning-after pill (chemical structure presented in Fig. 1a), is a synthetic progestogen presenting structural similarities with progesterone (chemical structure presented in Fig. 1b), it belongs to the second generation of progestins [1]. The differences between those active pharmaceutical ingredients (API) being observed at carbon 10 (the absence of the methyl group for levonorgestrel), carbon 13 (levonorgestrel presenting an ethyl group, while progesterone a methyl group) and at carbon 17 (levonorgestrel exhibiting two radicals, namely ethynyl and hydroxyl, while progesterone one acetyl radical). Those differences in the chemical structures of the two APIs determine two distinctive biopharmaceutical profiles: progesterone presents a very low oral biodisponibility (due to the significant hepatic first pass metabolism), while for levonorgestrel it is greater than 90% [2, 3]. Also, due to the particular radicals grafted to the structure, levonorgestrel binds distinctive to the human steroid receptors in vitro, as follows: an increased selectivity for the progesterone receptors and a lower selectivity for the androgen, glucocorticoid and mineralocorticoid receptors [1]. These particularities causing a considerable decrease in the number of adverse reactions, thus increasing the patient’s compliance with the treatment.
Levonorgestrel is mostly used for its contraceptive effect in the form of intrauterine devices, subcutaneous implants, patches, dragées, tablets, coated tablets and orodispersible tablets being the first contraceptive that was used containing only a derivative of progestogen [4, 5]. In spite of the fact that, initially, the daily dose, administered oral, to achieve the contraceptive effect was 0.030 mg. Lately, several studies demonstrated that the inhibition of ovulation takes place in only 72% of the cycles, suggesting the necessity for an adjustment of the therapeutic dose [6,7,8]. In consideration of this aspect, a certain amount of studies were performed demonstrating that the optimal dose for the contraceptive effect is 0.135 mg per day, a dose at which the interindividual variation is reduced to minimum [8,9,10]. The dosage for emergency contraceptive is 1.5 mg once or two doses of 0.75 mg at 12 h apart [2, 11]. Beside the use of levonorgestrel in progestogen-only pills, it is used in association with ethinylestradiol in combined oral contraceptive pill. The main advantages of combined oral contraceptive pill containing levonorgestrel, when compared to the ones, which contain progestins from the third generation (desogestrel and gestodene), are the considerable decrease in the risks of pulmonary embolism, ischemic stroke, myocardial infarction and venous thromboembolism [12, 13].
Because of its pharmacological properties, wide use and various pharmaceutical formulations, it is necessary to evaluate the presence of any potential interactions between the API and pharmaceutical excipients in the development of new formulations which possess increased stability and shelf life. Despite the fact that pharmaceutical excipients are considered inert substances added to the formulation, there is a considerable number of papers describing the interaction between the API and the excipients [14,15,16,17].
Excipients are classified according to their role in a pharmaceutical formulation, as follows: binders, disintegrants, diluents, lubricants, glidants, compression aids, colors, sweeteners, preservatives, flavors, film formers and suspending/dispersing agents/surfactants [18, 19]. The interactions between the API and the excipients can be classified as physical (complexation, adsorption, solid dispersion) and chemical interactions (hydrolysis, oxidation, racemization, polymerization, Maillard reactions, photolysis) [18, 20]. In order to identify the possible interactions between the API and excipients, the following analytical methods of analysis can be used: isothermal stress testing (analysis using HPLC), differential scanning calorimetry (DSC) and differential thermal analysis (DTA), isothermal microcalorimetry, powder X-ray diffraction, Fourier transform Infrared spectroscopy, solid state nuclear magnetic resonance spectroscopy, scanning electron microscopy, hot stage microscopy, diffuse reflectance spectroscopy, TLC and HPTLC [20,21,22,23].
The purpose of this study was to establish if there are any interactions between levonorgestrel and eleven pharmaceutical excipients, namely: colloidal silicon dioxide, aspartame, microcrystalline cellulose, sodium carboxymethyl cellulose, calcium lactate pentahydrate, α-lactose monohydrate, mannitol, polyvinylpyrrolidone K30, starch, magnesium stearate and talc. The analytical methods, which were employed, are: thermal analysis (TG/DTG/DSC), Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) and powder X-ray diffraction (PXRD). For a more accurate interpretation of the obtained results, statistical calculations were performed, namely obtaining the value of the correlation coefficient.
Materials and methods
Samples and preparation
Levonorgestrel (LNG) was obtained from Sigma–Aldrich (St. Louis, MO, USA), batch no.: 86302520. The purity of the active principle was according to the British Pharmacopoeia Reference Standard. The excipients used are: colloidal silicon dioxide (SiO2, batch no.: 3157040314, from Degussa AG., Germany), aspartame (ASP, batch no.: W700655, Sigma, Germany), microcrystalline cellulose (CelMC, #MKBR5799V, from Sigma, Germany), sodium carboxymethyl cellulose (NaCMC, #MKBT6160V, from Sigma, Germany), calcium lactate pentahydrate (CaL, batch no.: C8356, Sigma, Germany), α-lactose monohydrate (Lact·H2O, #SLBK4809V, from Sigma, Germany), mannitol (MNT, #BCBM1675V, from Sigma, Germany), polyvinylpyrrolidone K30 (PVP, batch no.: #BCBV7579, from Sigma, Germany), starch (St, SZBF167, from Grain Processing Corporation, USA), magnesium stearate (MgS, #SZBF2590V, from Sigma, Germany) and talc (T, #BCBQ7164V, from Sigma, Germany). The excipients were used without any further purification and were suitable to be used for medical purposes.
For testing the compatibility of LNG with each pharmaceutical excipient, eleven physical binary mixtures (BM) were prepared by weighing 50 mg of LNG and 50 mg of each excipient, corresponding to the 1:1 (w/w) ratio, in an agate mortar, triturated for 5 min and then placed in a sealed brown vial at 25 °C, in absence of light. Also, LNG and the eleven excipients were triturated and used for the analytical investigations.
Thermal investigations
To acquire the TG and DTG curves and study the thermal stability of LNG, excipients and the BM the NETZSCH TG 209 F1 Phoenix (NETZSCH, Selb, Germany) instrument and open alumina crucibles were used. The curves were recorded under non-isothermal conditions with a heating rate of β = 10 °C min−1 from room temperature (30 °C) to 600 °C, in dynamic air atmosphere (20 mL min−1). The calibration of the TG instrument was made with In, Sn, Bi, Zn, Al and Au. To obtain the DSC curves a NETZSCH DSC 204 F1 Phoenix (NETZSCH, Selb, Germany) instrument and sealed aluminum crucibles were used in inert nitrogen medium with a flow rate of 20 mL min−1, from 40 to 250 °C, with a heating rate of β = 10 °C min−1. The calibration of the DSC instrument was made with C10H16, In, Sn, Bi, Zn and CsCl.
ATR-FTIR investigations
In order to obtain the FTIR data for LNG, excipients and BM, a Bruker Vertex 70 spectrometer with ATR (Bruker Optics Gmbh, Ettlingen, Germany) instrument was used. The selected spectral range was between 4000 and 400 cm−1. The spectra were built up after 32 co-added scans. A theoretical spectrum was built for the BM, by using the data from the pure active pharmaceutical ingredient and the corresponding excipient. Then, the theoretical spectra were compared with the experimental ones, by using the Pearson’s correlation in different spectral regions, the excluded regions were 4000–3600 cm−1, 3200–3000 cm−1 and 2700–1700 cm−1, as in those regions no absorption band was observed for LNG or for the selected excipients, except for aspartame, where the 1800–1700 cm−1 was considered, and for talc, where the 3700–3600 cm−1 region was used, as in both cases for these excipients relevant absorption bands were noticed. The statistical calculations were performed on the MedCalc (version 20.015) statistical software. A value of the Pearson’s correlation between 1.00 and 0.80 signifies the lack of chemical interactions between the two components of the BM, a value found in the interval of 0.80 and 0.50 may indicate that a interaction between LNG and the excipient is possible and a lower value than 0.50 indicates the lack of similarity between the two compared spectra, suggesting the presence of a chemical interaction [24, 25].
PXRD investigations
To obtain the results of the PXRD investigations a Rigaku Ultima IV instrument operating at 40 kV and 40 mA was used and the diffractograms were recorded using the monochromated CuKα radiation (λ = 1.5406 Ao). For the statistical calculations a theoretical diffractogram was built using the data from the pure active pharmaceutical ingredient and corresponding excipient, then compared with the experimental diffractogram. To determine the value of the Pearson’s correlation for each selected region the MedCalc (version 20.015) statistical software was used. A correlation value between 1.00 and 0.80 indicates the lack of chemical interactions between the two components of the BM. If the value is found in the interval of 0.80 and 0.50, the results may illustrate that a chemical interaction between LNG and the excipient is possible and a value lower than 0.50 indicates a lack of similarity between the two compared diffractograms, suggesting the presence of a chemical interaction.
Results and discussion
Thermal investigations
The results of the thermal analysis are presented in Table 1 and as well in Figs. 2–4 where the TG, DTG and DSC curves for LNG, excipients and BM are drawn.
The results of the thermal analysis show a thermal stability of LNG up to 185 °C and a decomposition process that takes place in three steps. In the first step, LNG is losing more than half of its mass. The endothermic peak with an enthalpy of fusion of 141.4 J g−1, observable on the DSC curve at 243 °C describes the melting point of LNG, the value being in accordance with the data presented on PubChem (232–239 °C) [26].
In order to establish if an interaction between the API and the excipient is present the results of the thermal analysis are very valuable, especially the ones from the DSC investigations. In the absence of an interaction the DSC curve of the BM should look as a sum of the DSC curves of the API and excipient. The existence of a physical interaction is shown as changes in the intensity of the peaks of each component of the binary mixture. If on the DSC curve can be observed a significant shift in the melting of the components or appearance of a new exothermic or endothermic peak it can be concluded that between the API and the excipient a chemical interaction is present [20]. Also, in the case of a chemical interaction observed on the DSC curve, the enthalpy of fusion characteristic to the melting point of LNG should be less than half of the enthalpy of fusion obtained for the pure active pharmaceutical ingredient. In the case of the TG curves, considering the fact that for the BM the ratio between LNG and the excipient is 1:1 (w:w), each mass loss characteristic to each component of the physical mixture should be observed on the TG curve of the BM, the expected loss of mass being half of the one of the pure compound. For the DTG curves, all the decomposition processes noticed for the pure compounds, should be found on the DTG curve of the BM.
For SiO2 the TG, DTG and DSC curves do not show any significant event under the thermal conditions used in this study. The TG and DTG curves for the BM formed between LNG + SiO2 shows an increase in the stability for LNG up to 195 °C. The decomposition process is taking place in 3 steps. Considering that for the physical mixture the ratio between API and excipient is 1:1 (w:w), for the first decomposition process (which can be associated with the first process of LNG), the expected mass loss is 29.06%, but in this case it is less, also suggesting the increasing of stability for LNG. The DSC curve for the BM shows an endothermic peak at 235 °C, which is at a lower temperature than the one of the API. Also, the enthalpy of fusion (50.61 J g−1) is less than half of the expected one. Those aspects leading to the hypothesis of a possible chemical interaction between LNG and SiO2 when heated.
When studying the thermoanalytical data for ASP, it can be observed that its stability is up to 150 °C and the process of decomposition is divided into 3 steps. The DSC curve shows two endothermic peaks at 132 °C and 187 °C. In the case of the BM formed between LNG and ASP the results obtained are very different in comparison with the ones of the pure substances. The first process observed on the DTG curve of the mix (131–185 °C) can be correlated with the first decomposition step of ASP (150–213 °C) showing a decrease in its stability. Also, for the BM the second step of the decomposition begins at 154 °C and can be associated with the first step of LNG and the second one for ASP, demonstrating the decreasing of the stability of LNG in the presence of ASP. The DSC curve shows three endothermic peaks at 166, 209 and 226 °C, the ones for ASP at a higher temperature and for LNG at a lower temperature. These results demonstrating the existence of a chemical interaction between LNG and ASP.
The TG and DTG curves of CelMC describe a thermal stability up to 270 °C and a decomposition process with one step. The DSC curve presents two peaks at 31 and 81 °C. The physical mixture between LNG and CelMC’s first decomposition process begins at 209 °C, showing an increase in the stability of the API. The DSC for the mix presents a peak at 242 °C, but the intensity of it, when compared to the one of LNG, is half (enthalpy of fusion being 66.63 J g−1), describing the existence of a physical interaction between LNG and CelMC.
For NaCMC the decomposition starts at 30 °C and is a three-step process. The DSC curve shows two endothermic peaks at 32 °C and 84 °C. The first decomposition step of the BM between LNG and NaCMC starts at 30 °C, but for the second step at 187 °C, demonstrating a slight increase in the thermal stability of LNG. The DSC curve for the BM presents two endothermic peaks at 79 and 242 °C. The second peak (characteristic to the melting point of LNG) has half of the intensity of the one of LNG, as pure substance, namely 67.81 J g−1. Those aspects suggest that between LNG and NaCMC exist a physical interaction.
The decomposition process of CaL starts at 30 °C, with the loss of the five molecules of water, and consists in a three steps process. On the DSC curve an endothermic peak can be observed at 88 °C. In the case of the physical mixture, the loss of the excipients water molecules begins at 30 °C and the degradation process being one in five steps. The second process observed on the DTG curve, which characterizes the first process for LNG and the second one of CaL, commenced at 200 °C demonstrating an increase in the thermal stability of LNG. The DSC curve presents two endothermic peaks at 96 and 241 °C, with a slightly difference of the temperature observed for the pure substances. In order to establish the existence of an interaction further analysis are required. The half intensity peaks for CaL and LNG observed on the DSC curve of the BM (ΔHfus = 62.11 J g−1 for LNG) when compared to the data obtained for the pure substances indicate the presence of a physical interaction between those two.
Lact·H2O decomposes in four steps, the first one begins at 95 °C due to the loss of the water molecule. The DSC curve shows three endothermic peaks at 147, 220 and 239 °C. In the case of the BM formed between LNG and Lact·H2O the second decomposition process, characteristic to the first decomposition step of LNG and the third and fourth processes of Lact·H2O, starting at 201 °C, shows an increase in the thermal stability of LNG. On the DSC curve of the BM three endothermic peaks are observed at 147, 216 and 239 °C, with half of the intensity of the pure API of the enthalpy of fusion. The thermoanalytical results demonstrate the lack of a chemical interaction between LNG and Lact·H2O and the presence of a physical interaction between those two.
The results of the thermal analysis (TG and DTG) for MNT show a unique decomposition process and the DSC curve one endothermic peak at 174.8 °C. For the BM the endothermic peaks observed on the DSC curve (168 and 237 °C) presents a small shifting in comparison with the ones for LNG and MNT as pure substances, implying the existence of a possible interaction with the active substance. Also, the enthalpy observed for the endothermic peak characteristic to LNG is slightly higher than expected, supporting the hypothesis of a chemical interaction between those two. On the DTG curve of the BM the first decomposition process can be associated with the process of MNT and the first process of LNG, presenting two peaks at 303 and 330 °C. These observations lead to the hypothesis of a possible interaction between MNT and LNG, which need to be furthermore analyzed.
The PVP decomposition takes place in two steps, the first one being represented by dehydration. The DSC curve shows a broad endothermic phenomenon, with a peak at 75 °C. On the DTG curve of the BM four steps of the decomposition process can be observed, the one for the API being in the temperature range of 212–357 °C, showing an increasing in the thermal stability of the API. On the DSC curve of the BM a new endothermic peak at 182 °C appears, also the endothermic peak associated with the melting point of LNG is shifted to a lower temperature (226 °C) and the enthalpy of fusion being significantly reduced. Thus, the results of the thermal analysis demonstrate the existence of a chemical interaction between LNG and PVP.
On the TG and DTG curves of St in the temperature range 30–141 °C takes place the dehydration of the pharmaceutical excipient, followed by the decomposition process between 228 and 346 °C. The dehydration phenomenon can be, also, observed on the DSC curve as an endothermic process with the peak at 105 °C. The physical mixture between LNG and St presents a four steps decomposition process. The first one being associated with the dehydration of St and is correlated with an endothermic peak on the DSC curve (80 °C). The second decomposition step (temperature range 200–375 °C) is associated with the decomposition of St and the first step decomposition of LNG, where can be noticed that there is a slight increase in the thermal stability of LNG. The intensity of the endothermic peaks on the DSC curve is half in comparison with the one of the pure excipient and API, that is due to the physical interactions that exist between LNG and St. The shift of the endothermic peak characteristic to the dehydration of St leads to the hypothesis of a possible chemical interaction between LNG and St, which need to be furthermore analyzed.
MgS decomposes in a three steps process, the first one being the result of several dehydration stages with a mass loss of ~ 3%. Those stages are, also, shown as two endothermic peaks on the DSC curve at 91 and 116 °C. The third phenomenon observed on the DSC curve is an exothermic peak 165 °C. Regarding the physical mixture formed between LNG and MgS the results of the thermoanalytical investigations are very different. On the TG and DTG curves of the BM it can be observed that the decomposition process for LNG starts at 195 °C, which is at a slightly higher temperature showing an increase in the thermal stability of LNG. On the DSC curve is noticed the appearance of a new endothermic peak (126 °C), along with a significant shifting of the melting point of LNG (233 °C) and a reduction of the enthalpy of fusion. These results demonstrate the presence of a chemical interaction between LNG and MgS.
The TG, DTG and DSC curves for T do not show any significant event under the thermal conditions used in this study. The TG and DTG curves of the BM describe a three steps decomposition process, the first one starting at 195 °C demonstrating a light improvement in the thermal stability of LNG. Also, the DSC curve of the BM presents one endothermic peak at 241.6 °C, with a lower intensity (ΔHfus = 69.05 J g−1) than the one of LNG. Considering those results, between LNG and T exist only a physical interaction.
ATR-FTIR investigations
The results of ATR-FTIR investigations are presented in Table 2 and Fig. 5 shows the FTIR spectra for LNG, the BM and the excipients. The wavenumbers written in bold for the BM signify the appearance of new absorption bands on the spectra when compared to the ones of the API and the excipient. In Figure S1 can be observed the results of the statistical calculations, the regions for which the correlation value was lower than 0.80 being the ones where differences between the theoretical and experimental spectra can be observed.
The results of the ATR-FTIR investigations were interpreted according to the data presented in the scientific literature [27, 28]. The wavenumbers of each absorption band observed on the LNG spectrum could be correlated with the functional groups and chemical bonds present in the chemical structure of the API, demonstrating the identity and the degree of analytical purity. In order to confirm the presence of an interaction between the API and the excipient used to obtain the BM on the spectra of the BM, when compared to the ones of the API and the excipient, there has to be some shifting in the absorption bands of the API and the excipient or the appearance/missing of some peak. The disadvantage of this method is the presence of overlapping peaks in the spectra, which may hinder the analysis [20]. In addition, to obtain a more accurate evaluation of a possible chemical interaction at ambient temperature the Pearson’s correlation value was determined for each spectral region.
The first large absorption band present on the ATR-FTIR spectra of LNG in the spectral region of 3526–3277 cm−1, with maxim at 3342 cm−1, is due to the stretching vibrations of the dimeric hydroxyl group. In addition, the stretching vibration of the bond formed between carbon and the oxygen from the hydroxyl group is shown at the wavenumber equal to 1067 cm−1. For the carbonyl moiety the absorption band is shifted to a lower wavenumber, attributable to the delocalization of the π electrons of the unsaturated groups caused by the conjugation of the double bond, thus an intense absorption band is observed at 1651 cm−1. Furthermore for the C–C(= O)–C group on the spectra there are noticed two absorption bands, with moderate intensity, at 1211 and 1132 cm−1, being the result of the stretching of C–C–C and C–C(= O)–C bending in the C–C–C group. The low intensity band characteristic to the stretching vibrations of the monosubstituted terminal alkyne can be seen at 2164 cm−1, as well for the bond between carbon and hydrogen in this functional group a strong band is remarked at 3267 cm−1. For the same bond, the bending vibrations determine two absorption bands, with high intensity, at 692 and 656 cm−1, which are over toned by a weak broad band with maximum at 1232 cm−1. The stretching vibrations of the double moiety from the cycloalkene can be noticed at 1618 cm−1. The stretching vibrations of the methyl radical determines two intense peaks, the first one at 2932 cm−1 (for the symmetrical stretching) and the second one at 2868 cm−1 (for the asymmetrical stretching). Into the bargain the bending vibrations of the same group are observed at 1388 cm−1, the symmetrical ones, and at 1447 cm−1, the asymmetrical ones, usually it overlaps the scissoring vibration of the methylene groups, but in this case, due to conjugation, the band for this type of vibration is distinct at 1468 cm−1. The stretching vibrations for the C–H bond in the cycloalkanes appear at 2980 and 2932 cm−1, a slight shift is caused by the conjugation. The bending vibrations of the same bond are observed at 1458 cm−1.
By studying the ATR-FTIR spectra for the BM, in the case of the physical mixtures formed between LNG and CelMC, NaCMC, Lact·H2O and T the results of the thermal analysis are confirmed, namely the lack of a chemical interaction between the two components. For each of the four excipients the results of the Pearson’s correlation, confirmed the lack of a chemical interaction at ambient temperature. In the case of T the lower values for the Pearson’s correlations in the fingerprint region are due to absorption bands which do not appear on the theoretical spectra, but can be correlated with absorption bands noticed on the spectra of LNG. For CelMC and NaCMC the majority of the values of the correlations were in the range of 0.80 and 1.00, with one exception, caused by the difference in the intensity of the absorption bands. Regarding the BM between LNG and Lact·H2O the correlations values were close to 0.90, indicating the lack of differences between the theoretical and experimental spectra.
Regarding the BM formed between LNG and SiO2, on the spectra is noticed the disappearance of several absorption bands characteristic to the API and the excipient in all three spectral ranges. On the spectrum of SiO2 the broad band between 1303 and 875 cm−1 (maxim at 1082 cm−1), caused by the asymmetrical stretching vibrations of Si–O–Si moiety, determines a broad band in the spectral region of 1288–908 cm−1 of the BM (maxim at 1088 cm−1), which may hide the characteristic bands of LNG in this region. Considering that aspect and the results of the thermal analysis, more investigations are necessary in order to conclude the presence or the absence of a chemical interaction between LNG and SiO2. Thus, the statistical calculations performed for this mixture indicated a value of correlation close to 1.00, which indicates the lack of a chemical interaction between the two compounds at ambient temperature.
When comparing the ATR-FTIR spectra for the physical mixture formed between LNG and ASP with the ones of the pure substances it can be observed the absence of numerous peaks of the API and ASP, alongside with the appearance of new absorption bands. Those results correlated with the ones from the thermal investigations confirm the existence of a chemical interaction between LNG and ASP even at ambient temperature. Regarding the statistical calculations in the same regions where differences were observed, the values of the Pearson’s correlation are close to 0.80, which may indicate the possibility of a chemical interaction which needs to be furthermore analyzed.
In the case of the BM between LNG and CaL a few differences are noticed in the fingerprint region, leading to the hypothesis of a possible chemical interaction between the two components of the mixture. The results of the Pearson’s correlation support this hypothesis, being obtained relatively low values for r in the fingerprint region. Associated with the data obtained from the thermal analysis in order to confirm the chemical interaction it is required a new method of analysis, namely PXRD.
For the LNG + MNT mixture the data from the ATR-FTIR investigation do not show any interaction between the API and the excipient at ambient temperature. This conclusion leads to the hypothesis of a chemical interaction between LNG and MNT only when the mixture is heated. In order to confirm this assumption PXRD analysis will be employed. Those results are also sustained by the statistical calculations, the value of the Pearson’s correlation, in most spectral regions, being close to 0.80.
In regard to the spectrum obtained for the BM formed from LNG + PVP, there are major differences when compared to the spectra of LNG and PVP. The first major irregularity observed is the missing of the peak for the board absorption band between 3665 and 3022 cm−1 from the PVP spectra, along with the disappearance of the peaks present in the spectral range of 3026–2788 cm−1 and the peaks observed at the following wavenumbers: 1373, 1317, 1171, 1018 and 1001 cm−1. When compared to the spectra of LNG, the differences are noticed especially in the fingerprint region, lack of some peaks (1618, 1468, 1388, 1294 and 1088 cm−1) or the shifting of others (1447 → 1443 cm−1, 970 → 966 cm−1). Those aspects associated with the results of the thermal investigations demonstrate the possibility of a chemical interaction between LNG and PVP. Considering these results, the statistical calculations play a crucial role in establishing the presence of a chemical interaction. By employing Pearson’s correlation the theoretical and experimental spectra were almost identical (r = 0.98). In order to corroborate the presence or the absence of a chemical interaction at ambient temperature between the two components of the BM a further analytical investigation will be performed, namely PXRD.
Comparing the spectra of LNG with the one of the mixture between LNG and St, it can be noticed that all the peaks of the API are at the same wavenumber. The changes on the spectra of the BM are observable when it is overlaid with the one of pure St, namely: the missing of two board absorption bands between 3661 and 2403 cm−1 and 1064–945 cm−1, alongside with the lack of the peaks noticed in the fingerprint region of the St. These modifications may be caused by the low number of moles of St when compared to the ones of LNG in the mixture, the difference order being of 10−3 mol. The value obtained for the Pearson’s correlation, namely r = 0.96, suggested the lack of a chemical interaction between LNG and St. For a more detailed evaluation of a possible chemical interaction between the API and the excipient the PXRD investigation will be employed.
The results of the ATR-FTIR analysis for the BM between LNG and MgS confirm the conclusion of the thermal investigations, i.e., the existence of a chemical interaction between LNG and MgS. To confirm this theory, the ATR-FTIR spectra show the absence of numerous absorption bands for LNG (2932, 2855, 2833, 1468, 1458 cm−1) and MgS (2359, 1279, 1265, 1076 and 1018 cm−1) and appearance of new absorption peaks at the following wavenumbers: 2363, 2342 and 1113 cm−1. Considering the differences obtained, with the help of the statistical calculations, in the fingerprint region of the theoretical and experimental spectra, the hypothesis of a chemical interaction between LNG and MgS is sustained.
PXRD investigations
The results of PXRD investigations are presented in Table S1 and in Fig. 6 are shown the diffractograms of LNG, excipients and BM, respectively. The degrees written in bold for the BM signify the appearance of new peaks on the diffractogram when compared to the ones of the API and the pure excipient. In Figure S2 are presented the results of the statistical calculations, the regions for which the correlation value was lower than 0.80 being the ones where differences between the theoretical and experimental diffractograms can be observed.
In the case of an interaction between the API and the excipient on the PXRD diffractogram, it should observe the absence of some characteristic peaks of the API or for the excipient used or the appearance of new ones [20].
The results obtained from the PXRD diffractograms for the BM formed between LNG and CelMC, NaCMC, Lact·H2O and T are in agreement with the results from the thermal and ATR-FTIR investigations, namely the lack of a chemical interaction between the two components of the physical mixture. The statistical results showed a value of the correlation close to 1.00 for the BM between LNG and CelMC. In the case of the BM containing NaCMC the low correlation value in the first region is determined by the baseline’s noise. The deviations observed for Lact·H2O and T were the result of the difference in the intensity of the peaks. In the case of MNT the PXRD graph confirms the hypothesis of a chemical interaction between LNG and MNT only when heated and the absence of an interaction at ambient temperature. The same hypothesis is sustained by the results of the Pearson’s correlation, where all the peaks of the theoretical diffractogram can be observed on the experimental diffractogram, the only difference being in the peak’s intensity. The diffractogram for LNG + ASP, showed the appearance of new peaks, also rendered by the relatively low values of the Pearson’s correlation, demonstrating the presence of a chemical interaction between the two components of the mixture both during heating and at ambient temperature. For the mixture with PVP considering the shifting of some peaks, along with the results of the statistical correlation, namely r = 0.79, special precautions should be considered when associating the API with this excipient, due to the high risk of a chemical interaction between those two. In the case of the BM with MgS, the appearance of new peaks on the diffractogram, the relatively low values for the Pearson’s correlations and the changes noticed on the ATR-FTIR spectra, it can be concluded that a chemical interaction between LNG and the excipient is present, even at ambient temperature.
In the case of the BM formed between LNG and SiO2, the results of the PXRD analysis shows no shifting of the peaks characteristic of LNG, thus demonstrating the lack of a chemical interaction between those two, results sustained by the high value of the correlation coefficient, namely close to 1.00. However, the shifting of the melting point of LNG observed on the DSC curve for the BM and the lower mass loss for the first process observed on the TG and DTG curves of the BM, than the one expected, suggest the existence of a chemical interaction between LNG and SiO2 only when heated.
Regarding the physical mixture between LNG and CaL the PXRD diffractograms demonstrate the lack of chemical interactions in this BM. Conclusion is also confirmed by the value of the correlation coefficient, being close to 0.90, indicating a high similarity between the theoretical and experimental diffractograms. Thus, the only interaction observed for this mixture is the physical one, concluded from the DSC results.
When studying the diffractogram for LNG + St all the characteristic peaks of LNG can be found, demonstrating the lack of a chemical interaction in the mixture. The statistical calculations performed indicated a lower value of the correlation in the first region, which is determined by the baseline’s noise, no other differences being observed in the other regions. Therefore, in this BM the lack of a chemical interaction between the two compounds is observed.
Conclusions
In this article, the thermoanalytical profile of the contraceptive agent LNG and its compatibility with eleven pharmaceutical excipients were studied. In order to achieve that, binary physical mixtures, with a mass ratio of 1:1 (w:w), between the API and the excipients were prepared and each sample (LNG, the BM and the pure excipients) were analyzed. The analytical methods used in this study are: thermal investigations (TG, DTG and DSC), ATR-FTIR investigations and PXRD investigations. Also, for a more accurate interpretation of the obtained data from ATR-FTIR and PXRD investigations, statistical calculations were performed to obtain the value of the Pearson’s correlation.
The thermoanalytical results showed a thermal stability for LNG up to 185 °C and a decomposition process consisting in three degradation steps, in the first one losing more than half of its mass. The melting point of LNG, as described by the DSC curve, is 243 °C.
This study demonstrates the existence of chemical interactions between LNG and the following excipients, at ambient temperature and when heated: ASP and MgS. In the case of the BM containing LNG and the following excipients, a chemical interaction was noticed only when the mixture is heated: SiO2, MNT and St. Regarding the physical mixtures between LNG and CelMC, NaCMC, CaL, Lact·H2O and T no chemical interaction was present both at ambient temperature and when heated. As concerns the BM between LNG and PVP, precautions should be taken into consideration, due to the existence of chemical interactions when the mixture was heated, and the high risk of a chemical interaction at ambient temperature.
References
Benagiano G, Primiero FM, Farris M. Clinical profile of contraceptive progestins. Eur J Contracept Reprod Heal Care. 2004;9:182–93.
Kook K, Gabelnick H, Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception. 2002;66:73–76f.
Sitruk-Ware R, Bricaire C, De Lignieres B, et al. Oral micronized progesterone: bioavailability pharmacokinetics, pharmacological and therapeutic implications — a review. Contraception. 1987;36:373–402.
Vrettakos C, Bajaj T. Levonorgestrel. 2023.
Akkam Y, Omari D, Sallam A. Drug-excipient interactions: an overview on mechanisms and effects on drug stability and bioavailability. Ann Rom Soc Cell Biol. 2021;25:8402–29.
Korver T. A double-blind study comparing the contraceptive efficacy, acceptability and safety of two progestogen-only pills containing desogestrel 75 μg/day or levonorgestrel 30 μg/day. Eur J Contracept Reprod Heal Care. 1998;3:169–78.
Rice CF. A comparison of the inhibition of ovulation achieved by desogestrel 75 μg and levonorgestrel 30 μg daily. Hum Reprod. 1999;14:982–5.
Duijkers IJM, Klipping C, Rautenberg T, et al. Effect on ovarian activity and ovulation inhibition of different oral dosages of levonorgestrel. Contraception. 2022;110:6–15.
Spona J, Schneider WHF, Bieglmayer C, Schroeder R, Pirker R. Ovulation inhibition with different doses of Levonorgestrel* and other progestogens: clinical and experimental investigations. Acta Obstet Gynecol Scand. 1979;58(S88):7–15.
Oettel M, Kochhar PS, Osterwald H, et al. The Progestin-Only-Pill (POP) is not a Niche option. Reprod Endokrinol. 2015;12:246–50.
Hansen LB, Saseen JJ, Teal SB. Levonorgestrel-only dosing strategies for emergency contraception. Pharmacotherapy. 2007;27:278–84.
Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:1–10.
Løkkegaard E. Low-dose second-generation oral contraceptives are associated with the lowest increased risk of cardiovascular adverse effects. Evid Based Med. 2016;21:232.
Niguram P, Polaka SN, Rathod R, Kalia K, Kate AS. Update on compatibility assessment of empagliflozin with the selected pharmaceutical excipients employed in solid dosage forms by thermal, spectroscopic and chromatographic techniques. Drug Dev Ind Pharmacy. 2020;46(2):209–18.
Nisar J, Iqbal M, Iqbal M, et al. Decomposition kinetics of levofloxacin: drug-excipient interaction. Z Phys Chem. 2020;234:117–28.
Daniel JSP, Cruz JC, Catelani TA, et al. Erythromycin-excipients compatibility studies using the thermal analysis and dynamic thermal infrared spectroscopy coupled with chemometrics. J Therm Anal Calorim. 2021;143:3127–35.
Lopes MS, Catelani TA, Nascimento ALCS, et al. Ketoconazole: compatibility with pharmaceutical excipients using DSC and TG techniques. J Therm Anal Calorim. 2020;141:1371–8.
Chaudhari SP, Patil PS. Pharmaceutical excipients : a review. Int J Adv Pharmacy Biol Chem. 2012;1:21–34.
Fathima N, Mamatha T, Qureshi HK, et al. Drug-excipient interaction and its importance in dosage form development. J Appl Pharm Sci. 2011;1:66–71.
Chadha R, Bhandari S. Drug-excipient compatibility screening-Role of thermoanalytical and spectroscopic techniques. J Pharm Biomed Anal. 2014;87:82–97.
Leyk E, Wesolowski M. The effect of cellulose derivatives on paracetamol crystallinity reduction. J Therm Anal Calorim. 2022;147:10037–48.
Calvino MM, Cavallaro G, Lazzara G, et al. Talc concentration effect on shelf life of acetaminophen tablets. J Therm Anal Calorim. 2023;148:13133–9.
Dichi E, Sghaier M, Guiblin N. Pharmaceutical phase diagram: aspirin-caffeine-paracetamol. J Therm Anal Calorim. 2023;148:6107–18.
Bezerra GSN, Pereira MAV, Ostrosky EA, et al. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J Therm Anal Calorim. 2017;127:1683–91.
De Barros Lima ÍP, Lima NGPB, Barros DMC, et al. Compatibility study of tretinoin with several pharmaceutical excipients by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120:733–47.
PubChem. Norgestrel, https://pubchem.ncbi.nlm.nih.gov/compound/Norgestrel. Accessed from 1 Oct 2023.
Silverstein RM, Webster FX, Kiemle DJ, et al. Spectrometric identification of organic compounds. 8th ed. NY: Wiley; 2014.
Stuart BH. Infrared spectroscopy: fundamentals and applications. Hoboken: Wiley; 2004.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by AR, AL, LS, CM and IL. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ridichie, A., Ledeţi, A., Sbârcea, L. et al. Preformulation studies of levonorgestrel. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13149-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13149-w