Abstract
This preliminary study employs differential scanning calorimetry (DSC) to assess alterations in human plasma composition in patients treated with cilostazol for intermittent claudication. Cilostazol, an antiplatelet and vasodilator agent, is commonly prescribed to moderate symptom associated with PAD caused by reduced peripheral blood flow. Patients selected according to study protocol were treated with cilostazol for a duration of three months. At the visits, subjects exercised by a standard walking test controlled by physiotherapist. The blood samples were taken on day 0 (control), day 15, 1, 2 and 3 months. Through DSC analysis, we investigate potential thermodynamic changes in plasma composition induced by cilostazol treatment. As a result of the treatment, the pain-free walking distance improved significantly in the first 3 months. The thermal unfolding of the human plasma showed a correlation with the walking improvement. Our findings provide insights into the systemic effects of cilostazol and its impact on plasma constituents in the context of PAD and intermittent claudication management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral arterial disease (PAD) is a common vascular disorder characterized by the narrowing or blockage of arteries supplying blood to the lower extremities. The clinical presentation of PAD can vary widely, ranging from asymptomatic cases to severe limb-threatening ischaemia. Intermittent claudication (IC), which means limitation of walking, is the first and most common manifestation of PAD caused by atherosclerosis [1, 2]. Patients with peripheral arterial disease have many therapeutic options, such as conservative therapy, surgical revascularization, or endovascular treatment. Complex conservative management of the patients with intermittent claudication includes regular walking exercise, risk factor modification (smoking cessation, healthy diet, mass loss, etc.) and pharmacotherapy. Due to the presence of generalized atherosclerosis, patients with intermittent claudication also are three times more likely to die of cardiovascular disease such as acute myocardial infarction (AMI) or STROKE, compared with patients without IC [3]. To decrease the higher cardiovascular mortality in PAD patients, lifelong antiplatelet and well-monitored statin therapy is highly appropriate. Pharmacotherapy alone is only used in the early stages of the disease, such as asymptomatic lower limb PAD (Rutherford grade 0/Fontaine stage I) and intermittent claudication (Rutherford grade I-III/Fontaine stage IIa and IIb).
Cilostazol is a phosphodiesterase III inhibitor that is primarily used for relief of intermittent claudication in patients with peripheral artery disease. [4] Cilostazol has multiple actions, including vasodilation and improvement of the vascular endothelium and microcirculation, in addition to antiplatelet activity. Several randomized clinical trials demonstrated that patients with intermittent claudication receiving cilostazol experienced a significant improvement in pain-free walking distance compared with those receiving placebo [5,6,7,8].
Aim of the study
Current research focused on the developing the application of DSC approach as a new monitoring method of cilostazol effects in patients with intermittent claudication. Differential scanning calorimetry is a widely used analytical technique that measures the heat flow into or out of a sample as a function of temperature or time. It provides valuable information about the thermodynamic properties of the sample, including phase transitions, thermal stability, and heat capacity changes. In summary, DSC is a well-established method for the demonstration of thermal consequences of local and global conformational changes in biological systems, but it has never been applied for the investigation of the human blood in PAD patients.
Patients and methods
For this study, only patients with intermittent claudication were considered. The Fontaine classification was used for categorization the patient’s symptoms. Inclusion criteria were age > 40 years and < 75 years old, controlled walking distance < 400 m and the absence of pedal pulses. Patients with serious ischaemic heart disease, chronic renal failure or limb-threatening ischaemia were excluded. Seven white adults (four men and three women; median age 58.6 years) enrolled into this preliminary study. All the patients except one were studied up to 3 months after enrolment. One patient (women) had femoral amputation in the following period (day 78.) because of late irreversible ischaemic injury of the right foot. All patients were taking aspirin (100 mg/day) and statins at the time of enrolment. During follow-up period, patients’ regular medication did not change. Subjects exercised by a standard walking test are controlled by physiotherapist. The distance when claudication pain was first perceived was recorded. Before the administration of cilostazol therapy, control blood sample was collected from all the patients. After general physical examination, the subjects rested for 30 min before undergoing venepuncture from an antecubital fossa vein with tourniquet. Control (without cilostazol therapy) peripheral blood samples were collected from the patients (n = 7) and from healthy control (n = 5). Each of the selected patients received 2 × 100 mg cilostazol daily. Patients were asked to return for clinic visit at 2 weeks, 1, 2, 3 months. At the visits, after general check-up, blood samples were taken, and walking distances have been controlled. No adverse drug reactions were observed in patients during the follow-up period. The study was approved by the Hungarian Medical Research Council (IV/2448.4/2022), and all patients gave their written consent to the investigations.
Blood sample preparation
Blood samples were collected into the Vacutainer tubes containing EDTA (1.5 mg mL-1 of blood) and centrifuged at 1600 g for 15 min at 4 °C to separate plasma fraction from cell components. Just after the centrifugation native plasmas undergone to a thermal denaturation made by SETARAM Micro DSC-III or were stored at 4 °C in water–ice mixture till the next DSC measurement.
DSC measurement
The thermal unfolding of the human plasma was monitored by SETARAM Micro DSC-III calorimeter as previously described [9, 10]. All experiments were conducted between 0 and 110 °C. The heating rate was 0.3 K min−1 in all cases. Conventional Hastelloy batch vessels were used during the denaturation experiments with 950 μL sample volume in average. Reference sample was normal saline (0.9% NaCl). The sample and reference samples were equilibrated with a precision of ± 0.1 mg. The repeated scan of denatured sample was used as baseline correction, which was subtracted from the original DSC curve. We have plotted the heat flow in the function of temperature. Calorimetric enthalpy was calculated from the area under the heat flow curve by using two-point setting SETARAM peak integration. The thermal data are given in average ± s.d., rounded to one decimal place for temperature and two decimal places for enthalpy.
Results and discussion
Following the three-month cilostazol treatment, the resulting values demonstrated a significant (15d p = 0,028, 1 m p = 0,016, 2 m p = 0,014, 3 m p = 0,011) increase in pain-free walking distance during all visits compared to the control measurements on the day 0. The 3-month data showed the most significant improvement in walking ability. Data were compared at baseline by using the analysis of two sample t test. One patient (women) had femoral amputation in the following period (day 78) because of late irreversible ischaemic injury of the right foot. It is important to mention that no adverse drug reactions were observed in patients during the follow-up period.
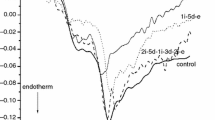
In Fig. 1, we present the summarized result of the whole denaturation experiment set.
The detailed thermodynamic parameters are given in Table 1.
It is obvious from the data that all average patient’s thermal parameter significantly differs from the healthy control. The starting stage of patients was worse than that of healthy ones. The increased denaturation temperature range refers to a less cooperative structure of patients’ plasma with circulatory disorders, which manifested also in the increased calorimetric enthalpy (see Table 1). During the medical treatment, all average thermal data remain in the range of mean ± 1 s.d. in case of control before treatment, but the patient’s data exhibited widespread. (From the treatment of 1 month only, ΔT and ΔHcal show a mild treatment time dependence.) This may be a sign of individual sensitivity to drug treatment, which could be determined by examining the various therapeutic drugs given for this disease and the individual responses to them, thus optimizing the individualized treatment from the point of drug and time of curing.
The most relevant alteration in thermal parameter of treated patients compared to the healthy control is the change in the running of DSC scans and the significant increase in ΔCp between native and denatured state of plasma. (More heat was supplied (absorbed) to the sample holder than into the control vessel, to follow the heating programme with no temperature difference between them). This can be the sign of the abnormal blood circulation because of the more densely “packed” plasma. The treatment of one and two months shows a significant decrease, indicating an improvement in the blood circulation. Comparing this parameter in between the treatment process, the patients before or after 2 weeks and 3 months of treatment exhibit a same heat capacity change. It was very surprising that it returned to the same value as the control was after three months, when we had to stop this investigation. It cannot be ruled out that this is related to the therapeutic drug’s mechanism of action, so new drugs must also be assessed. To clarify this, we need to monitor a larger number of patients and longer duration of drug treatment. Of course, it has some unexpected complication because the patients can fall out from the test period due to different personal life problems (e.g. unexpected, other type of illness that prevents the test) or surgery to prevent worsening of their condition.
Despite of the above-mentioned cases, according to our thermal results, we can see the applicability of DSC to monitor the effect of drug treatment in these medical situations. This is also confirmed by the painless journey test performed at different time of the drug treatment (see Fig. 2 and Table 2).
As shown in Fig. 2 and Table 2, there is a positive correlation between the effect of medical treatment and the completed pain-free walking distance, despite of the small number of treated persons.
Conclusions
Peripheral arterial disease is a prevalent vascular disorder characterized by atherosclerotic plaque formation in the arteries, leading to reduced blood flow, particularly in the lower extremities. Intermittent claudication, a distressing symptom of PAD, manifests as pain or discomfort in the lower leg during physical activity. The treatment goals for patients with IC include symptomatic relief and reduction the associated cardiovascular risk factors. Standard conservative management of patients with intermittent claudication includes a walking exercise, smoking cessation, antiplatelet and statin therapy, evaluation for associated cardiovascular disease, and drug treatment for symptomatic relief [11]. Surgical or endovascular intervention usually reserve for patients with severe, lifestyle-limiting walking problems, rest pain or non-healing peripheral wounds and gangrenes. In the conservative treatment, Cilostazol has emerged as a valuable treatment modality for PAD patients experiencing intermittent claudication. By enhancing blood flow, alleviating pain, and improving exercise tolerance, cilostazol contributes significantly to enhancing the quality of life for individuals affected by this chronic vascular condition. In our study, the significant increase in pain-free walking distance observed after a three-month cilostazol treatment aligns with previous research demonstrating the positive effects of cilostazol on peripheral blood flow and exercise tolerance in patients with intermittent claudication. The thermodynamic changes detected by DSC in the human plasma could attributed to various mechanisms associated with cilostazol’s mode of action. Vasodilation induced by cilostazol may lead to improved blood flow and oxygen delivery to the muscles, potentially affecting the thermal behaviour of plasma constituents. Additionally, cilostazol’s antiplatelet effects may influence plasma composition and subsequently impact its thermodynamic properties. Further research, a larger sample size and longer following period need to validate these findings and establish a more definitive link between the thermal properties of plasma constituents and the efficacy of cilostazol treatment. Additionally, exploring the relationship between DSC-measured plasma thermodynamics and other clinical parameters, such as ankle-brachial index (ABI) and quality of life assessments, could provide a comprehensive understanding of cilostazol’s impact on the systemic physiology of PAD patients.
Data availability
There are no additional available data to upload.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:1056–528.
Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–21.
Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–5.
Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003;3:117–38.
Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002;90:1314–9.
Liu Y, Shakur Y, Yoshitake M, Kambayashi JJ. Cilostazol (Pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and aden- osine uptake. Cardiovas Drug Rev. 2001;19:369–86.
Bradbury AW. The role of cilostazol (Pletal) in the management of intermittent claudication. Int J Clin Pract. 2003;57:405–9.
Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomized controlled trial. J Vasc Surg. 2007;45:65–70.
Ferencz A, Lőrinczy D. DSC measurements of blood plasma on patients with chronic pancreatitis and operable and inoperable pancreatic adenocarcinoma. J Thermal Anal Calorim. 2017;127:1187–92.
Ferencz A, Szatmári D, Lőrinczy D. Thermodynamic sensitivity of blood plasma components in patients afflicted with skin, breast and pancreatic forms of cancer. Cancers. 2022;14:6147.
Darvin H, King T. Lower extremity arterial occlusive disease. Clin Podiatr Med Surg. 1992;9:69–77.
Acknowledgements
This work was supported by CO-272 OTKA grant (DL).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
LB involved in rising the problem, sample preparation and handling, data analysis, and manuscript writing. GF involved in sample preparation and handling. DSz involved in sample preparation and handling. DL involved in corresponding author, principal investigator, DSC experiments, data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Benkő, L., Fazekas, G., Szabó, D. et al. Differential scanning calorimetric evaluation of the human plasma in patients using cilostazol for the treatment of intermittent claudication (a preliminary study). J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13167-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13167-8