Abstract
The ammonium sulphate roasting process involves reacting mineral-bearing materials with ammonium sulphate via a solid–solid roasting process and subjecting the resulting roast residue to aqueous leaching. This process enables the simultaneous, non-selective co-extraction of strategic metals from the starting materials. However, effective separation of the extracted metals is often mandatory to produce quality products of high purity. In this study, the combined application of thermogravimetric analysis, X-ray powder diffraction and inductively coupled plasma optical emission spectrometry confirmed the non-selectivity of the process when applied to a South African diamond mine residue residue roasted with ammonium sulphate in a 1:2 mass ratio (m/m) at 450 °C for 2 h, with magnesium, iron and aluminium being co-extracted into water-soluble metal sulphates. Thermogravimetry was then applied to develop a multi-step, multi-temperature selective roasting process using mixtures of pure commercial metal sulphate salts. The first step of the modified process successfully separated iron and aluminium sulphates from magnesium-sulphates in the roast residues by thermally decomposing soluble iron and aluminium sulphates into insoluble oxides via calcination at 750 °C for 2 h. This temperature was lower than the one at which magnesium sulphates convert into magnesium oxide. In the second and final step, iron and aluminium were recovered from the oxide minerals via solid–solid re-roasting with ammonium sulphate at 450 °C for 1 h, causing the oxides to revert back to their water-soluble sulphate forms. The effectiveness of the modified process was subsequently verified using a diamond mine residue, showing that the soluble iron and aluminium contents in the magnesium-bearing leachate could be reduced by over 90%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mining sector is integral to the South African economy, yet its operations result in the production of substantial amounts of waste mine residues such as tailings and slimes. These residues may pose significant environmental issues and occupy extensive land that could be utilised for alternative economic purposes. On the other hand, if processed into valuable products, these same residues may have the potential to be an economic resource that promotes sustainability.
The ammonium sulphate ((NH4)2SO4) roasting process is an emerging technology for the purification of silicate minerals (e.g. talc; [1]) and the extraction of strategic metals from mineral-bearing materials [2]. The latter includes materials such as coal fly ash [3,4,5], laterite nickel ore [6], platinum group metals (PGMs) tailings [7, 8], slags [2, 9], bauxite residue [10], serpentinite rocks [11,12,13], and other residues [14,15,16]. The basic principle of the process relies on the use of the thermochemical reactivity between (NH4)2SO4 (as chemical additive) and reactive oxide or silicate minerals to convert the insoluble metal components into their respective water-soluble metal sulphates and/or intermediate double salts (i.e. ammonium-metal sulphate-based compounds (NH +4 –M-SO 2−4 ). This step is followed by aqueous leaching in water or dilute acid to dissolve the sulphate compounds and extract the valuable metals to solution. This process has a number of benefits, such as low corrosion of equipment (in comparison to acid leaching using strong acids), high metal recovery, and the use of a widely available, low-cost, recyclable chemical agent (NH4)2SO4.
The success of the (NH4)2SO4 roasting process depends greatly on the silicate lattice structure of minerals contained in the parent material. For instance, minerals such as cordierite (the only Mg-bearing cyclosilicate) [11, 17] and talc (a phyllosilicate) [1, 11] have limited to no reactivity with (NH4)2SO4 during solid–solid thermal treatment. On the other hand, the magnesium contained in the two phyllosilicate minerals vermiculite and phlogopite is highly reactive with (NH4)2SO4 during roasting [11]. Serpentine minerals display varying degrees of reactivity with (NH4)2SO4, with lizardite pairing the favourable features more often than antigorite [12, 17, 18], even though the two minerals lizardite and antigorite are both Mg-rich 1:1 trioctahedral layer minerals with an ideal composition of Mg3Si2O5(OH)4. This is because minerals’ behaviour during the (NH4)2SO4 roasting process is mostly guided by their mineralogical structure, their parent rock, and any possible transformation the rock may have undergone through metamorphosis or other type of alteration [11].
A challenging aspect associated with the (NH4)2SO4 roasting process, experienced by the authors when reprocessing coal fly ash [4] and PGM tailings [7], is its lack of metal selectivity during the thermochemical step. This non-selectivity leads to the simultaneous co-extraction of several elements (e.g. Al, Ca, Mg, Fe and Ti) to solution due to the chemical affinity between (NH4)2SO4 and major elements under thermal conditions. Similar observations were made in other studies. For example, Zhang et al. [19] revealed the simultaneous extraction of Fe and Mn along with the elements of interest (V, Ti) from vanadium slag. Romão et al. [20] observed the co-extraction of Fe, Ni, Cr, Cu along with the element of interest (Mg) from serpentinite.
Effective separation of the extracted dissolved elements can be problematic (e.g. Al and Fe, Ca and Mg), although it is often essential to produce quality products of high purity. Increasing the roasting temperature to selectively convert some water-soluble metal sulphates and/or intermediate double salts into their respective insoluble metal oxides can successfully separate metals, although examples in the literature are limited. An example is that copper and nickel can be separated by decomposing ammonium ferric sulphate to iron oxides by increasing the roasting temperature from 450 to 500 °C following initial roasting of mixed oxide-sulfide nickel ore [21]. In a study by Saba et al. [22], where (NH4)2SO4 was used to extract Mn from a low grade ore containing 27.5% Fe, it was observed that the soluble Fe content and therefore its extraction decreased at roasting temperatures above 700 °C. These findings aligned with those of Meng et al. [23] in their study of Sc extraction from bauxite ore, where no Fe was extracted at temperatures above 650 °C. Fekete et al. [24] proposed a process for separation of a mixture of ZnSO4 and Fe2(SO4)3, derived from the sulphation of a ZnFe2O4 waste material. This involved annealing at 400–450 °C for extended durations or heat shock treatment between 600–650 °C, resulting in the conversion of Fe2(SO4)3 into Fe2O3. ZnSO4, being thermally stable at these temperatures, can be separated from the water-insoluble Fe2O3 by dissolving it in water. More recently, Kamberović et al. [25] demonstrated the selective extraction of Cu, Pb and Zn from a sulphate-rich jarosite tailing waste by converting the Fe-sulphate phases to hematite through roasting at 730 °C.
The objective of this paper is three-fold. Firstly, the study confirms the hypothesised non-selectivity of the (NH4)2SO4 roasting process when applied to a South African diamond mine residue. Secondly, the study uses thermogravimetry to develop a multi-step, multi-temperature selective roasting process using mixtures of pure commercial metal sulphate salts as models of solid residues generated via the (NH4)2SO4 roasting process. Finally, the effectiveness of the selective process is tested on the diamond mine residue.
Experimental
Starting material and chemicals
Ammonium sulphate ((NH4)2SO4) and deionised water were AR grade and were sourced from Merck Chemicals (Pty) Ltd. AR-grade metal sulphate salts (aluminium sulphate octadecahydrate (Al2(SO4)3·18H2O), iron(III) sulphate hydrate (Fe2(SO4)3·xH2O) and magnesium sulphate heptahydrate (MgSO4·7H2O)) were supplied by Labchem, South Africa. The three hydrated metal salts were placed in a muffle furnace at 400 °C for 2 h to remove the crystallisation water and produce their anhydrous forms, i.e. Al2(SO4)3, Fe2(SO4)3 and MgSO4.
A sample of mine slime (SL) was obtained from a mine in South Africa. The particles were finely grained, with the percentile diameters D(v,0.1), D(v,0.5) and D(v,0.9) calculated from laser diffraction volume data being 2.0 μm, 9.6 μm and 161.6 μm, respectively.
Methodology
Non-selective metal co-extraction from mine slime using conventional roasting with (NH4)2SO4
The control experiment consisted of subjecting 8.0 g of the mine slimes sample (SL) to roasting with 16.0 g of (NH4)2SO4 in a 1:2 mass ratio (m/m) in a muffle furnace at 450 °C for 2 h, to generate a roast product (SLAS) containing water-soluble metal sulphate compounds formed from the thermochemical reaction between reactive minerals and (NH4)2SO4. SLAS was then leached in deionised water using a ratio of 1:25 mass to volume (m/v), i.e. g mL−1, under continuous mixing condition at 50 °C for 2 h. After leaching, the suspension was filtered through a Sartorius polycarbonate Track-Etch membrane. The leach residue (SLAS-L) was washed with deionised water and air-dried. The acidified filtrates were characterised using ICP-OES. This leaching-filtration-washing-drying procedure is hereafter referred to as the “leaching method” and was used on all roast products generated in this study.
Selective metal extraction method development using synthetic metal sulphate salt mixture
TGA studies
The three anhydrous metal sulphate salts MgSO4, Fe2(SO4)3 and Al2(SO4)3 were finely ground using a mortar and pestle. The individual salts were then combined to produce a salt mixture (hereafter called “mixture” or MX) with a similar mass ratio to that of the mine slime sample (SL) (i.e. Mg:Fe:Al at 3:2:1). The mixture served as an analog to the solid residue SLAS generated following roasting of the mine slime with (NH4)2SO4. The thermal behaviour and decomposition temperature ranges of each individual sulphate salt were studied using TGA. The mixture was also investigated for direct comparison because the decomposition temperatures of the individual metal sulphates may shift considerably when combined together with other metal sulphate species [26]. The insight gained from the TGA data was then used to develop a multi-stage roasting-leaching regime for the selective extraction of Mg from Fe and Al contained in the mixture.
Selective roasting-leaching regime
In order to test the findings derived from the TGA data, the following roasting-leaching regime was developed. MX was first roasted at either 450 °C or 750 °C for 2 h. According to the TGA results obtained for MX, roasting at 750 °C was expected to convert Fe and Al sulphates into insoluble Fe and Al oxides. At completion of the roasting treatments, the roast products MX450 and MX750 were leached using the leaching method described earlier. The leach residue obtained from roasting the mixture at 750 °C (MX750-L) was roasted with (NH4)2SO4 in a 1:2 m/m ratio at 450 °C for 1 h and the new roast residue (MX750-AS) was leached to yield a new leach residue(MX750-AS-L). The roasting regime applied to the mixture, and the purpose of each roasting step, is summarised in Fig. 1 and Table S1 (Supplementary material).
Evaluation of the selective roasting-leaching regime using mine slimes
The effectiveness of the selective roasting-leaching regime was evaluated by preparing a new batch of SLAS following the procedure described in section "Non-selective metal co-extraction from mine slime using conventional roasting with (NH4)2SO4". Once SLAS was formed, it was roasted at 550 °C, 680 °C or 750 °C to form the SLAS-550, SLAS-680 and SLAS-750 roasts, respectively. According to the TGA results, it was anticipated that roasting SLAS at these three temperatures would convert Fe sulphates into insoluble Fe oxides to separate Fe from Al and Mg via selective thermal conversion of water-soluble Fe sulphates into insoluble Fe oxides to yield roast products SLAS-550 and SLAS-680, or to convert both soluble Al- and Fe sulphates into the corresponding insoluble oxides while retaining the Mg as soluble MgSO4 in SLAS-750. SLAS-680 was then leached using the leaching method to dissolve Mg and Al and form the leach residue SLAS-680L. SLAS-680L was subsequently roasted with (NH4)2SO4 at 450 °C to convert insoluble Fe into soluble Fe sulphates, yielding SLAS-680-AS. Roasting of SL at 680 °C with (NH4)2SO4 without the initial 450 °C roast was performed to reduce furnace time and to ascertain whether a single-step process would adequately separate the Fe phase from the Mg and Al phases. This roast product was labeled SLAS high. The roasting regime applied to the mine slimes, and the purpose of each roasting step, is summarised in Fig. 2 and Table S2 (Supplementary material).
Characterisation of solid materials and leachates
Solid materials
Chemical compositions of solid materials were obtained using XRF fused bead analysis (Thermo Fisher Perform’X Sequential XRF with OXSAS software). The loss on ignition (LOI) was determined by roasting the sample at 1000 °C for at least 3 h until a constant mass was obtained. Mineralogical compositions were obtained using a PANalytical X’Pert Pro Powder Diffractometer (XRD) equipped with an X’Celerator detector and variable divergence- and fixed receiving slits, with Fe filtered Co-Kα radiation (λ = 1.789 Å). Mineral phase concentrations were determined by Rietveld quantitative analysis with Highscore software with accuracy in the region of ± 1%. The samples were micronised in a McCrone micronising mill and prepared for XRD analysis using a back loading preparation method.
TGA analyses were performed on a TA Instruments SDT Q600 Thermogravimetric Analyzer & Differential Scanning Calorimeter (DSC). Approximately 20 mg of sample was placed in a 90-μL alumina pan and heated from ambient to 1100 °C at a heating rate of 10 °C min−1. Each test was conducted using a flow rate of 100 mL min−1 of nitrogen (N2).
Leachates
All leachates were acidified to a pH below 2 with concentrated nitric acid and analysed for their major element content by inductively coupled plasma optical emission spectrometry (ICP-OES) at an accredited laboratory (Waterlab Pty Ltd, Pretoria, South Africa).
Results and discussion
Characterisation of mine slime (SL)
The bulk chemical composition of SL is shown in Table 1. The sample was predominantly composed of SiO2, MgO, Fe2O3, Al2O3, CaO and TiO2 and exhibited a positive loss on ignition (LOI = 8.3%). The mineralogy of SL was complex (Table 2 and Figure S1 in the Supplementary material). It was dominated by phyllosilicate clay minerals (69% m/m), more specifically 33% smectite (most probably saponite or montmorillonite, or a mixture of both), 30% talc, 5% kaolinite and 1% lizardite. The abundance of these phyllosilicate clay minerals accounted for the large amount of SiO2 (47%, Table 1), and since many of these minerals contain magnesium, it also explained the substantial MgO content (24%, Table 1). Additionally, SL contained 8% phlogopite, 6% ferrian diopside, and various minor and trace minerals, each present in quanties less than 5% m/m. Based on these findings, Mg, Fe and Al were identified as the major elements available for extraction from SL. Si, even though the most abundant element, was not expected to be extractable using the process of solid-state reaction with (NH4)2SO4 [27]. In the context of this study, the selective extractive behaviour of Mg, Fe and Al was of particular interest.
Non-selective metal co-extraction from slime using conventional roasting with (NH4)2SO4
Conventional roasting of SL with (NH4)2SO4 was used to collect baseline data and demonstrate the non-selective co-extraction of major elements when using this process (Process 1, Fig. 2). Optimising the roasting temperature or the SL to (NH4)2SO4 ratio was outside the scope of this study. The baseline data were then used to assess the effectiveness of of the modified process.
The mineralogical characterisation of the roast product obtained from Process 1 (SLAS) by XRD indicated the occurrence of several newly formed metal sulphate phases (MgSO4.6H2O, (NH4)2Mg2(SO4)3, Al2(SO4)3, (NH4)Al(SO4)2 and NH4Fe(SO4)2) (Table 3 and Figure S1 in the Supplementary material). These results provided the first confirmation of the simultaneous extraction of several major elements (Al, Fe, Mg) from parent minerals present in the slime during the thermochemical step. The identification of Mg (2.3 g L−1), Fe (1.1 g L−1) and Al (0.5 g L−1) in the leachates following aqueous leaching of SLAS presented the second confirmation that elemental co-extraction had occurred.
Selective metal extraction method development using the pure metal sulphate mixture (MX)
TGA study
Table 4 summarises the decomposition reactions and theoretical TGA mass losses expected for the dehydrated pure metal sulphates and MX. Theoretical TGA mass losses were calculated from the stoichiometry of the decomposition reactions presented in the table. Figure 3a compares the decomposition behavior of the individual pure metal sulphates and MX.
The TGA mass losses obtained for dehydrated pure MgSO4 (67.0%; 900–1125 °C), Al2(SO4)3 (70.7%; 600–925 °C) and Fe2(SO4)3 (57.8%; 550–750 °C) were in agreement with the calculated values reported in Table 4. These mass loss values were calculated on a dry mass basis, with the temperature at 200 °C taken as the onset. The average of duplicate TGA measurements indicated that the mass losses and decomposition temperature ranges for MX were 19.9% between 550 °C and 680 °C (Fe2(SO4)3 → Fe2O3), 9.0% between 680–775 °C (Al2(SO4)3 → Al2O3) and 33.1% between 775 and 1030 °C (MgSO4 → MgO). The minimum in the DTG curve was used to assign temperature ranges. The mass losses obtained for decomposition of the metal sulphates contained in MX agreed well with the expected values (Table 4). A shift in decomposition temperatures for the metal sulphates in MX in comparison to that of the pure metal sulphates was clearly shown by the TGA results. All metal sulphates in MX had decomposition temperature ranges lower than those of the pure metal sulphates, and their decomposition events were not well separated, especially for the decomposition of Fe2(SO4)3 and Al2(SO4)3. The results obtained for the thermal stabilities of Fe2(SO4)3, Al2(SO4)3 and MgSO4 contained in MX are summarised in Fig. 4.
Development of selective roasting-leaching regime for MX
The insight gained from the TGA data was used to develop a multi-stage roasting-leaching regime for the selective extraction of Mg from Fe and Al contained in MX. Figure 4 illustrates that separation of Fe from Al and Mg in a mixture of their sulphate salts may be achieved by thermally treating the mixture at temperatures where water soluble Fe2(SO4)3 is decomposed to insoluble Fe2O3 while Al2(SO4)3 and MgSO4 are kept in the soluble sulphate form, i.e. between 550 and 680 °C. Similarly, if both Fe and Al are to be separated from Mg, thermal treatment of the mixture should occur at temperatures between 680 and 775 °C. Leaching of the thermally treated mixture is expected to yield the soluble metal sulphate in the leachate and the insoluble metal oxide in the solid leach residue. Re-roasting of any of the solid leach residues with (NH4)2SO4 at 450 °C is anticipated to convert the remaining insoluble metal oxides back into soluble metal sulphates, which can again be recovered by aqueous leaching.
In order to test the findings derived from the TGA data, MX was roasted at either 450 °C or 750 °C. The roasting regime applied to MX, and the purpose of each roasting step, is summarised in Fig. 1 and Table S1 (Supplementary material). Roasting at 450 °C was performed to confirm the thermal stability of the metal sulphates at the temperature used for thermochemical treatment of the mine residues with (NH4)2SO4. The roasting temperature for separation of Mg from Fe and Al was adjusted from 775 °C to 750 °C, using the temperature at which the rate of decompositon of Al2(SO4)3 contained in MX is at its maximum, i.e. the peak temperature in the DTG curve (Fig. 3a). This was done to limit the conversion of MgSO4 into MgO in an effort to retain most of the Mg in the leachate and all of the Fe and Al in the solid leach residue. This hypothesis was tested by repeating the TGA analysis of MX using a heating rate of 20 °C min−1 and inclusion of an isotherm at 750 °C for 30 min (Fig. 3b). Mass losses obtained for the decomposition of the pure metal sulphates Fe2(SO4)3 (21.0%), Al2(SO4)3 (10.5%) and MgSO4 (31.3%) were similar to the expected values (Table 4) and those obtained during the normal TGA ramp experiment (Fig. 3a). These results indicated that complete decomposition of Al2(SO4)3 can be attained when lowering the roasting temperature of MX from 775 to 750 °C, and that MgSO4 did not decompose during the isothermal step at 750 °C. Finally, the solid leach residue obtained from roasting MX at 750 °C was thermochemically treated with (NH4)2SO4 at 450 °C and leached in water to recover the Fe and Al.
TGA analysis of roast products MX450, MX750 and MX750L-AS is illustrated in Fig. 5. Curve 1 (MX450) showed that Mg, Fe and Al remained in the soluble sulphate form after oven roasting of MX at 450 °C. All three metals can therefore be extracted from MX450 by aqueous leaching. Curve 2 (MX750) was similar to that reported for pure MgSO4 (Fig. 3a), indicating that only Mg remained in the sulphate form after roasting MX at 750 °C. This was supported by the ICP-OES data (Fig. 6).
MX750L-AS was the product obtained from thermochemical treatment of the leach residue from the 750 °C roasting program (MX750-L) with (NH4)2SO4 (Fig. 1). The purpose of thermochemical treatment of MX750-L was to determine whether Fe and Al could be recovered from their respective oxides (Fe2O3 and Al2O3). The presence of Fe2(SO4)3 and Al2(SO4)3 in curve 3 (MX750L-AS) illustrated that both Fe and Al locked in oxide forms can be recovered as water-soluble metal sulphate forms after re-roasting of MX750-L with (NH4)2SO4. This result proves that the conversion of the metal sulphates to metal oxides is reversible when re-treating with AS at 450 °C. Decomposition of the Fe2(SO4)3 in MX750L-AS overlapped with that of Al2(SO4)3 between 580 and 830 °C, as indicated by the shoulder in the DTG curve. Excess (NH4)2SO4 is also apparent (400–550 °C, curve 3), as was observed previously for thermochemical treatment with (NH4)2SO4 at 450 °C for 1 h [1, 4, 8]. The rate of decomposition of Fe2(SO4)3 to Fe2O3, determined from the peak temperature in the DTG curves, was at its maximum at 700 °C for both MX450 and MX750L-AS. However, the onset of decomposition of Al2(SO4)3 occured below 700 °C, evident by the overlap in DTG curves between 600 and 800 °C (Fig. 5, curves 1 and 3). Efficient thermal separation of Fe2O3 from Al2(SO4)3 may therefore be challenging when applying a subsequent roast of MX at 700 °C. A TGA mass loss of 10.3%, indicating the temperature range over which 50% of Fe2(SO4)3 has decomposed, was noted for MX450 between 580 and 680 °C (Fig. 5, curve 1). This midway point for decomposition of Fe2(SO4)3 was selected as the roasting temperature to retain Mg and Al as soluble sulphates when applying this program to SL. This temperature was similar to the temperature reported by Meng et al. [23], i.e. ≥ 650 °C when working with bauxite residues, and was therefore selected as the roasting temperature to retain Mg and Al as soluble sulphates when applying this program to SL.
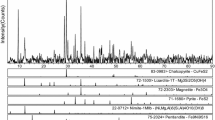
The proposed separation method was tested by aqueous leaching of MX450, MX750 and MX750L-AS (Fig. 6). Mg, Al and Fe were all extracted from leaching of MX450. A very small amount of dissolved Fe and Al (0.3% and 2.4% of the original amount, respectively) was measured in solution after leaching of MX750. The presence of Fe-oxides in the leach residue of MX750 (i.e. MX750-L) was evident by its dark red colour (Fig. 7). Particulate hematite is typically red in nature [28, 29]. This was supported by XRD data, which confirmed that hematite (Fe2O3) was the major Fe-containing phase in MX750-L, with strong reflections at 2θ of 28.5°, 38.5°, 41°, 48°, 64°, 74° and 76° (Fig. 7). Fe and Al were also successfully recovered and extracted from MX750L-AS, after re-roasting of MX750L at 450 °C with additional (NH4)2SO4, and no Mg was identified in the leachate (Fig. 6).
Application to the mine residues
The roasting program used for the mixture of pure metal sulphates was adapted and applied to the mine slimes sample. SL was initially roasted at 450 °C with (NH4)2SO4 to convert the minerals in SL to soluble metal sulphates (Process 1). Additional roasting at either 550 °C or 680 °C was conducted to retain Fe as insoluble Fe oxide in the leach residue, i.e. decrease the Fe content of the leachate, and retain Mg and Al as soluble sulphates (Processes 2 and 3). The rationale behind the inclusion of an additional roasting step at 550 °C is explained later in the discussion. Roasting at 750 °C was conducted with the intention to keep only Mg in soluble sulphate form (Process 4). An additional experiment, using roasting at 680 °C with (NH4)2SO4, without the initial 450 °C roast, was also undertaken (Process 5). This was to reduce furnace time and to ascertain whether a single step process would adequately separate the Fe phase from the Mg and Al phases. Finally, recovery of Fe from the leach residue obtained from Process 3 was tested by re-roasting the solid leach residue at 450 °C with (NH4)2SO4 (Process 6). These roasting programs and the purpose of each roasting step, are summarised in Fig. 2 and Table S2 (Supplementary material).
XRD analysis of the roasting products from Processes 1, 3 and 4 indicated the presence of ammonium-metal sulphate and metal sulphate phases containing Mg, Al and Fe after roasting of SL at 450 °C with (NH4)2SO4 (Table 3, Process 1 and Figure S1 in the Supplementary material). The ammonium-metal sulphate phases were absent following roasting at 680 °C or 750 °C (Table 3, Processes 3 and 4). At these higher roasting temperatures, MgSO4 and Al2(SO4)3 were observed as the only phases containing Mg and Al, while Fe2O3 was the only form of Fe present.
Figure 8 compares the TGA curves obtained from Process 1, i.e. the product of thermochemical treatment of SL with (NH4)2SO4 (SLAS) to that of the mixture of pure sulphate salts (MX). For SLAS (curve 2), the decomposition of Fe2(SO4)3 to Fe2O3 occurred between 425 and 550 °C, Al2(SO4)3 to Al2O3 between 550 and 750 °C, and MgSO4 to MgO between 750 and 980 °C, all of which were considerably lower than for the mixture of pure chemicals (curve 1). This shift in decomposition temperatures may be attributed to numerous factors such as differences in particle size [25], variations in structural order, the presence of impurities [25], or even the presence of other phases such as talc and hornblende in the mine residues. Furthermore, this paper reports a shift in the decomposition temperatures of the metal sulphates in MX when compared to that of the pure metal sulphates (Fig. 3a). To account for the shift in the decomposition temperature ranges of the metal sulphates contained in SL, an additional program involving roasting at 550 °C was incorporated to facilitate the separation of Fe from Al and Mg (Fig. 2, Process 2). In comparison, the roasting program applied in the separation of Fe from Al and Mg in MX, which included additional roasting at 680 °C, was also implemented for SL (Fig. 2, Process 3).
Figure 9 illustrates the TGA results obtained for the roasting products after applying the roasting programs to SL. The absence of a mass loss between 425 and 550 °C in curve 2, obtained for SLAS-550, indicated that the Fe2(SO4)3 phase was decomposed during the additional roasting step at 550 °C. Decomposition of Al2(SO4)3 (550–750 °C) and MgSO4 (800–980 °C) was still observed. The TGA result obtained for SLAS-680 (curve 3) indicated that the Fe2(SO4)3 phase was decomposed, but the mass loss due to decomposition of the Al2(SO4)3 phase has also been reduced substantially (from ~ 13 to ~ 5%) when the temperature of the additional roasting step was increased to 680 °C. This temperature (680 °C) was higher than the temperature indicated as the cut-off for decomposition of Fe2(SO4)3 in SLAS (curve 1), for which Fe2(SO4)3 was mostly decomposed at about 550 °C, while decomposition of Al2(SO4)3 already commenced. It was therefore anticipated that leaching of SLAS-550 and SLAS-680 will not yield Fe in solution, the extraction of Al from SLAS-680 should be lower than from SLAS-550, and the Mg concentrations should be similar. TGA analysis of SLAS-750, the solid product obtained from roasting SL with (NH4)2SO4 at 450 °C followed by additional roasting at 750 °C, indicated that only MgSO4 was retained in the roasted product (curve 4). The Fe2(SO4)3 and Al2(SO4)3 phases were fully decomposed following roasting at 750 °C. Aqueous leaching of SLAS-750 was therefore expected to yield only Mg in solution.
The extent of Mg, Al and Fe extraction from SL subjected to the various roasting programs and subsequent leaching in water was followed by ICP-OES analysis, reported as elemental concentration (Fig. 10a) and percentage elemental extraction from SL (Fig. 10b). The values for maximum possible extraction were determined from the XRF data (Table 1), excluding the Mg content of the 30% m/m talc phase present in SL (Table 2). A prior study indicated that talc exhibits minimal, if any, reactivity during thermochemical treatment with (NH4)2SO4 under experimental conditions similar to this study [1].
Leaching of the product from Process 1 (SLAS) yielded Mg (2.3 g L−1), Fe (1.1 g L−1) and Fe (0.5 g L−1) in the leachate (Fig. 10a). More than 90% of extractable Mg and approximately 65% of total Al and Fe were leached from SLAS (Fig. 10b, Process 1). The ICP-OES results of the leachates supported the reduction in the Al content predicted by TGA analysis of the solid products obtained from Processes 2 and 3, i.e. after roasting SL at 450 °C with (NH4)2SO4 followed by an additional roast at either 550 °C or 680 °C. However, Fe continued to be extracted even when performing the additional roast at 680 °C, despite the absence of visible decomposition of Fe2(SO4)3 in curves 2 and 3 (Fig. 9). The soluble Fe content of the leachate for Process 3 was, however, decreased by 75–79% (Process 1 vs. Process 3; Fig. 10). Additional roasting at 550 °C did not negatively impact the extraction of Al, but increasing the roast temperature to 680 °C resulted in a substantial loss of Al. These results indicate that separation of Fe from Al, without substantial loss of Al, remains a challenge. The leachate obtained from Process 4 primarily contained Mg, with minimal Fe and Al still being extracted. The Fe and Al contents in this leachate were reduced by ca. 90% compared to those from Process 1. It suggests that the effective separation of Mg from Al and Fe can be achieved by incorporating an additional roasting step at 750 °C.
Mg and Al extracted from Process 5 (78.4% and 28.7%;; Fig. 10b) were lower than when using Process 3 (97.3% and 36.4%; Fig. 10b), whereas the Fe extraction was slightly higher (15.0% vs 12.6%; Fig. 10b), indicating that the initial roast with (NH4)2SO4 at 450 °C to convert the Mg- and Al-containing phases in the SL to soluble sulphates should not be omitted.
Fe was successfully recovered from Process 6, where the leach residue from Process 3 (SLAS-680L) was re-roasted with (NH4)2SO4. The leachate was enriched in Fe with a concentration of 1.5 g L−1, while Mg and Al occurred in concentrations of approximately 0.5 g L−1 (Fig. 10a). This process led to the recovery of almost 90% of the Fe content from SL (Fig. 10b).
The solid leach residues obtained from Processes 1, 3 and 4 were analysed via semi-quantitative XRD to confirm the efficient dissolution of sulphate minerals during the leaching process and to identify potential application for these residues (Table 5). Talc was the predominant phase in all these residues, constituting over 52%, a substantial increase from the 30% talc content in SL. The talc residue obtained from Process 1 appeared as a creamy white product, compared to the original grey colour of SL. Given its improved colour clarity, this residue may hold promise for direct application in the ceramics, paint or paper industries. The occurrence of the hornblende and diopside phases can be reduced by using the additional roasting regimen at higher temperatures, which could lead to a residue which is further enriched in talc content (e.g. Process 3, Table 5). The presence of hematite (Fe2O3) increased at higher roast temperatures, corresponding to the reduction in leached Fe during Processes 3 and 4 (Fig. 10). Although some of these residues had a slightly higher talc content, their brick-red colour (similar to the image displayed for MX750-L in Fig. 7) may limit their suitability for application in the aforementioned industries. The absence of sulphate minerals in the residues confirmed the efficiency of the leaching process.
Conclusions
-
Thermochemical treatment of South African diamond mine residues with (NH4)2SO4 followed by aqueous leaching was a non-selective process, which resulted in the simultaneous extraction of Mg, Al and Fe into solution.
-
Separation of Mg from Fe and Al was efficiently achieved using a selective roasting program prior to leaching.
-
Fe was selectively removed from the leachates using a sequence of systematic roasting steps, although the temperature ranges used require further testing and refinement. The remaining Fe in the leach residue was successfully recovered from the solid leach residues by re-roasting the residue with (NH4)2SO4 to convert the oxide into the soluble sulphate form. The challenge of separating Fe from Al, without substantial loss of Al, remains.
-
The Mg-rich leachate from Process 4 may be used to produce pure Mg(OH)2 by precipitation with ammonia solution. The synthesis of Mg/Al or Mg/Fe layered double hydroxides (LDHs) can be pursued from the leachates obtained from the remaining processes, as a combination of these divalent and trivalent cations are required for its synthesis. The solid leach residue obtained following processes 1 and 6 could be considered for applications in the ceramic, paint or paper industry since it is enriched in talc and benefits from an improved colour clarity.
-
Thermogravimetry played a crucial role as research tool during the method development for the separation of these elements from the mine residue sample. It was used to identify and quantify the soluble sulphates formed during thermochemical treatment and to determine the decomposition temperature ranges of metal sulphates.
References
Castleman BA, Van Der Merwe EM, Doucet FJ. Thermochemical purification of talc with ammonium sulphate as chemical additive. Miner Eng. 2021;164: 106815. https://doi.org/10.1016/j.mineng.2021.106815.
Ju J, Feng Y, Li H, Xu C, Xue Z, Wang B. Extraction of valuable metals from minerals and industrial solid wastes via the ammonium sulfate roasting process: a systematic review. Chem Eng J. 2023;457: 141197. https://doi.org/10.1016/j.cej.2022.141197.
Wu Y, Xu P, Chen J, Li L, Li M. Effect of temperature on phase and alumina extraction efficiency of the product from sintering coal fly ash with ammonium sulfate. Chin J Chem Eng. 2014;22(11–12):1363–7. https://doi.org/10.1016/j.cjche.2014.09.008.
Doucet FJ, Mohamed S, Neyt N, Castleman BA, van der Merwe EM. Thermochemical processing of a South African ultrafine coal fly ash using ammonium sulphate as extracting agent for aluminium extraction. Hydrometallurgy. 2016;166:174–84. https://doi.org/10.1016/j.hydromet.2016.07.017.
Ma Y, Stopic S, Xakalashe B, Ndlovu S, Forsberg K, Friedrich B. A cleaner approach for recovering Al and Ti from coal fly ash via microwave-assisted baking, leaching, and precipitation. Hydrometallurgy. 2021;206: 105754. https://doi.org/10.1016/j.hydromet.2021.105754.
Li J, Li Y, Duan H, Guo X, Zhai Y. Experimental and kinetic study of magnesium extraction and leaching from laterite nickel ore by roasting with ammonium sulfate. Russ J Non-Ferr Met. 2018;59(6):596–604. https://doi.org/10.3103/S1067821218060123.
Mohamed S, Lehong K, van der Merwe EM, Altermann W, Doucet FJ. Thermochemical treatment of platinum group metal tailings with ammonium salts for major element recovery. J Therm Anal Calorim. 2019;138(3):2015–33. https://doi.org/10.1007/s10973-019-08233-5.
Mohamed S, van der Merwe EM, Altermann W, Doucet FJ. Process development for elemental recovery from PGM tailings by thermochemical treatment: preliminary major element extraction studies using ammonium sulphate as extracting agent. Waste Manag. 2016;50:334–45. https://doi.org/10.1016/j.wasman.2016.02.021.
Lin Q, Zhang G, Wang K, Luo D, Tang S, Yue H. Two-stage cyclic ammonium sulfate roasting and leaching of extracting vanadium and titanium from vanadium slag. Chin J Chem Eng. 2022;47:39–47. https://doi.org/10.1016/j.cjche.2021.05.035.
Borra CR, Mermans J, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner Eng. 2016;92:151–9. https://doi.org/10.1016/j.mineng.2016.03.002.
Lavikko S, Eklund O. The role of the silicate groups in the extraction of Mg with the ÅA route method. J CO2 Util. 2016;16:466–73. https://doi.org/10.1016/j.jcou.2016.10.012.
Nduagu E, Björklöf T, Fagerlund J, Wärnå J, Geerlings H, Zevenhoven R. Production of magnesium hydroxide from magnesium silicate for the purpose of CO2 mineralisation—part 1: application to Finnish serpentinite. Miner Eng. 2012;30:75–86. https://doi.org/10.1016/j.mineng.2011.12.004.
Nduagu EI, Highfield J, Chen J, Zevenhoven R. Mechanisms of serpentine–ammonium sulfate reactions: towards higher efficiencies in flux recovery and Mg extraction for CO2 mineral sequestration. RSC Adv. 2014;4(110):64494–505. https://doi.org/10.1039/C4RA08925A.
Deng L, Qu B, Su S-J, Ding S-L, Sun W-Y. Extraction of iron and manganese from pyrolusite absorption residue by ammonium sulphate roasting-leaching process. Metals. 2018;8(1):38. https://doi.org/10.3390/met8010038.
Zhu H, Sun Q, Yan J, Zhang J, Sheng J. Recycling of municipal sewage sludge incineration fly ash based on (NH4)2SO4 roasting-acid leaching and filling PP matrix process. Environ Sci Pollut R. 2022;29(60):89986–95. https://doi.org/10.1007/s11356-022-22061-5.
Ballou I, Kounbach S, Naja J, Bakher ZE, Laraki K, Raibi F, Saadi R, Kholtei S. A new approach of aluminum extraction from drinking water treatment sludge using ammonium sulfate roasting process. Miner Eng. 2022;189: 107859. https://doi.org/10.1016/j.mineng.2022.107859.
Lavikko S, Eklund O. The significance of the serpentinite characteristics in mineral carbonation by “the ÅA Route.” Int J Miner Process. 2016;152:7–15. https://doi.org/10.1016/j.minpro.2016.04.009.
Sjöblom S, Eklund O.Suitability of Finnish mine waste (rocks and tailings) for Mineral Carbonation. In: Zevenhoven R editor Efficiency, cost, optimization, simulation and environmental impact of energy systems (ECOS 2014). Conference proceedings; 15–19 June 2014; Turku, Finland; 2014.
Zhang G, Luo D, Deng C, Lv L, Liang B, Li C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J Alloys Compd. 2018;742:504–11. https://doi.org/10.1016/j.jallcom.2018.01.300.
Romão I, Gando-Ferreira LM, Zevenhoven R. Separation and recovery of valuable metals extracted from serpentinite during the production of Mg(OH)2 for CO2 sequestration. Miner Eng. 2015;77:25–33. https://doi.org/10.1016/j.mineng.2015.02.003.
Mu W, Cui F, Huang Z, Zhai Y, Xu Q, Luo S. Synchronous extraction of nickel and copper from a mixed oxide–sulfide nickel ore in a low-temperature roasting system. J Clean Prod. 2018;177:371–7. https://doi.org/10.1016/j.jclepro.2017.12.260.
Saba AE, Hussein MK, Khairy EM. Study on the sulphatisation of a local low grade manganese ore. Egypt J Chem. 1973;16(6):529–40.
Meng F, Li X, Shi L, Li Y, Gao F, Wei Y. Selective extraction of scandium from bauxite residue using ammonium sulfate roasting and leaching process. Miner Eng. 2020;157: 106561. https://doi.org/10.1016/j.mineng.2020.106561.
Fekete F, Lázár K, Keszler AM, Jánosity A, Zhibin L, Szilágyi IM, Kótai L. Recycling the industrial waste ZnFe2O4 from hot-dip galvanization sludge. J Therm Anal Calorim. 2018;134(3):1863–72. https://doi.org/10.1007/s10973-018-7849-8.
Kamberović Ž, Ranitović M, Manojlović V, Jevtić S, Gajić N, Štulović M. Thermodynamic and kinetic analysis of jarosite Pb–Ag sludge thermal decomposition for hydrometallurgical utilization of valuable elements. J Therm Anal Calorim. 2023;148(21):11799–810. https://doi.org/10.1007/s10973-023-12508-3.
van der Merwe EM, Strydom CA. Quantitative thermogravimetric analysis of binary mixtures. J Therm Anal Calorim. 2004;76(1):149–56. https://doi.org/10.1023/B:JTAN.0000027814.93703.0c.
Bayer G, Kahr G, Mueller-Vonmoos M. Reactions of ammonium sulphates with kaolinite and other silicate and oxide minerals. Clay Miner. 1982;17(3):271–83. https://doi.org/10.1180/claymin.1982.017.3.01.
Georgiev P, Ivanova M-F, Nicolova M, Spasova I, Groudev S. Review of the iron recovery processes from laden leach liquors in hydrometallurgy. Sustain Extrac Process Raw Mater J. 2020;1:31–6. https://doi.org/10.58903/a14161825.
Voynick S. Rock science: the colors of hematite. In: Rock&Gem; 2018. https://www.rockngem.com/rock-science-the-colors-of-hematite/. Accessed 5 Dec 2022.
Acknowledgements
The authors thank Ms Wiebke Grote for XRD, Ms Jeanette Dykstra for XRF and the University of Pretoria Laboratory for Microscopy and Microanalysis for assistance with FESEM. The authors thank the Council for Geoscience and FJD for providing supervisory assistance, insight and expertise. The project was financially supported by the University of Pretoria and the National Research Foundation of South Africa (NRF) (Grant Numbers: 117944 and 138020). Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
Funding
Open access funding provided by University of Pretoria.
Author information
Authors and Affiliations
Contributions
B.A.C., F.J.D. and E.M.v.d.M. contributed to the study conception and design. Material preparation, data collection and analyses were performed by all authors. The first draft of the manuscript was written by B.A.C., F.J.D. and E.M.v.d.M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castleman, B.A., Doucet, F.J., Roos, L. et al. Thermogravimetry as a research tool for the development of an ammonium sulphate roasting process for selective metal extraction from minerals. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13151-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13151-2