Abstract
Some thermophysical properties such as densities, speeds of sound, refractive indices, and heat flows of the binary systems ethyl levulinate and n-alkanols (methanol, ethanol, 1-propanol, and 1-butanol) are reported in the temperature range T = (283.15–313.15) K and at p = 100 kPa. From these experimental data, the excess properties were calculated and correlated with the composition using a modified Redlich–Kister equation. The excess molar volumes were negative for short-chain alcohols and positive for 1-propanol and 1-butanol. The excess refractive indices exhibited the opposite behaviour. The excess isentropic compressibilities were negative and the excess molar enthalpies, were positive. The perturbed chain statistical associating fluid theory equation of state (PC-SAFT EoS) was successfully applied to correlate the densities of the mixtures, and good qualitative results in the computation of the excess molar enthalpy were obtained. Furthermore, Schaaff’s collision factor theory (SCFT) and the Laplace mixing rule were coupled with PC-SAFT to predict the speed of sound and the refractive index, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the middle of the last century, the use of biomass-derived fuel has been growing exponentially owing to the current awareness of the multiple environmental, economic, and social problems caused by the dependence on fossil fuels [1]. Because of these properties, ethyl levulinate can be used as a biofuel.
In this study, we present the density, speed of sound, and refractive indices of four systems containing ethyl levulinate and the first four linear alkanols at three different temperatures (283.15, 298.15, and 313.15 K) and at pressure, p = 100 kPa. Furthermore, the excess molar enthalpies of the four systems were obtained under identical temperature and pressure conditions. Studying the thermophysical properties of liquid mixtures is important because of their use in several industries and chemical processes [2]. In previous papers, we presented a similar study containing methyl levulinate [3] instead of ethyl levulinate and also presented the surface behaviour of binary systems involving alkyl levulinates and alcohols [4].
We found two papers in the literature reporting the thermophysical properties of the ethyl levulinate system with ethanol at several temperatures and at atmospheric pressures. Ramly and Abdullah [5] reported densities, refractive indices, and surface tensions at six temperatures T = (298.15–323.15) K. Majstorović et al. [6] reported densities, speeds of sound, refractive indices, and dynamic viscosities in the same temperature range.
To model the results, the perturbed chain statistical associating fluid theory equation of state (PC-SAFT EoS) [7, 8] was used for liquid density and excess molar enthalpy. In addition, the speed of sound and refractive index were modelled correctly using Schaaff’s collision factor theory (SCFT) [9] and the Laplace mixing rule [10], respectively.
Experimental
Table 1 lists the chemicals used. Water content was measured using a Crison KF 1S-2B automatic titrator. No additional chemical purifications were performed.
Here, we only mention the equipment used to perform the experimental measurements and their uncertainties, as a detailed description and calibration can be found in previous studies [3, 11].
The density, ρ, and speed of sound, u, were determined using an Anton Paar DSA 5000. Both cells, density cell and sound velocity cell are temperature-controlled by a built-in Peltier thermostat. The uncertainties for temperature, density, and speed of sound, were 0.005 K, 10–4 g cm−3, and 1 m s−1, respectively.
The refractive index of the sodium D-line (wavelength λ = 589.3 nm), nD, was measured using an Abbemat-HP Dr. Kernchen refractometer. The corresponding uncertainties were for refractive index 10–4 and 0.01 K for temperature.
Mixtures were prepared by mass using a CP225-D Sartorius Semimicro balance with a precision of 10–5 g. The corresponding estimated uncertainty in mole fraction was 10–3.
Excess molar enthalpy, \(H_{{\text{ m}}}^{\text{E}}\), was determined using a Thermometric 2277 thermal activity monitor working under both isothermal (within ± 2 × 10–4 K) and constant flow conditions [12]. Two calibrated Shimadzu LC-10ADVP HPLC pumps were employed to drive the liquids to the calorimeter, and the uncertainty in the mole fraction was 0.01. Calibration was performed [11] using the highly accurate \(H_{{ \text{m}}}^{\text{E}}\) of the n-hexane system with cyclohexane [13]. The uncertainty in the excess molar enthalpy was 1%.
Table 2 shows the experimental values of density, speed of sound, and refractive index of the chemicals at the three temperatures and atmospheric pressure and those reported in the literature at T = 298.15 K and atmospheric pressure [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. In addition, the values of the isobaric heat capacities [29, 30] and isobaric expansivities are included. The isobaric expansivities were obtained in our laboratory. In this comparison, the only deviation is the speed of sound of ethyl levulinate; the deviation between our value and the one reported by Majstorović et al. [6] is 2.14 m s−1, making our value a little bit higher.
Results and discussion
Table S1 of the Supplementary Material reports the experimental results for density, ρ, speed of sound, u, and refractive index, nD, along with the calculated isentropic compressibility. The isentropic compressibility, κS, can be obtained from ρ and u values using the equation: \(\kappa_{{ \text{S}}} = {1 \mathord{\left/ {\vphantom {1 {{(}\rho \, u^{2} {)}}}} \right. \kern-0pt} {{(}\rho \, u^{2} {)}}}\), supposing negligible ultrasonic absorption.
From the experimental thermophysical properties of the mixtures—density, isentropic compressibility, and refractive index, and pure compound thermophysical properties—in the same physical state as the mixture [31], the corresponding excess properties can be calculated using adequate equations [32, 33]. The pure compound properties required to calculate the excess isentropic compressibility, isobaric molar heat capacity, and isobaric expansivity are listed in Table 1.
here, ρ represents mixture density, ρi represents pure compound densities, xi represents mole fractions of the components, Mi represents corresponding molar masses, nD is the mixture refractive index, nD,i are the pure compound refractive indices, and ϕi, are volume fractions of the components referred to the unmixed state.
With regard to the excess molar enthalpy, \(H_{{\text{ m}}}^{\text{E}}\), can be determined directly from the compensating heating power and molar flux of the components.
The excess properties, such as excess molar volume, excess isentropic compressibility, and excess refractive index, are reported in Table S1 of the Supplementary Material, and the excess molar enthalpy is included in Table S2. These excess properties are plotted as a function of composition in Figs. 1–3.
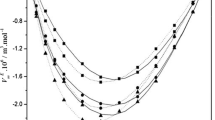
Excess properties as a function of the mole fraction, x1, or the volume fraction, ϕ1, at T = 283.15 K and at p = 100 kPa for the binary mixtures ethyl levulinate (1) + n-alkanol (2): (black square) methanol; (black circle) ethanol; (white square) 1-propanol; (white circle) 1-butanol; (straight line) Redlich–Kister equation
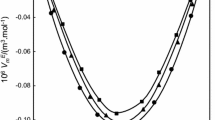
Excess properties as a function of the mole fraction, x1, or the volume fraction, ϕ1, at T = 298.15 K and at p = 100 kPa for the binary mixtures ethyl levulinate (1) + n-alkanol (2): (black square) methanol; (black circle) ethanol; (white square) 1-propanol; (white circle) 1-butanol; (straight line) Redlich–Kister equation
Excess properties as a function of the mole fraction, x1, or the volume fraction, ϕ1, at T = 313.15 K and at p = 100 kPa for the binary mixtures ethyl levulinate (1) + n-alkanol (2): (black square) methanol; (black circle) ethanol; (white square) 1-propanol; (white circle) 1-butanol; (straight line) Redlich–Kister equation
The obtained excess properties were correlated with the composition (mole or volume fraction) using a modified Redlich–Kister polynomial expansion [34] this modification was proposed by Heintz et al. [35]:
QE is the excess property, z1 ethyl levulinate's the mole or volume fraction, and Ai and Bj are adjustable parameters. The best fitting parameters and standard deviations, σ(QE), are listed in Table 3.
Table 4 reports the absolute average relative deviation, AARD between experimental values and those reported in the literature for the binary mixture containing ethanol at temperatures 298.15 and 313.15 K:
Our density values are somewhat lower than those reported by Ramly and Abdullah [5]. When comparing our densities with the values reported by Majstorović et al. [6], we observed that some of our values are negative, while the bulk are positive. However, in this case, the deviations are small. Additionally, our refractive index values are somewhat lower than those reported by Ramly and Abdullah [5]. The comparison of refractive index values with those reported by Majstorović et al. [6] exhibits similar trends to those observed in the case of comparing density values, with some comparative values being negative and some positive. However, in this case, the deviations are small. Finally, our speeds of sound are mostly higher than those of Majstorović et al. [6], particularly at high concentrations of ethyl levulinate.
The excess molar volumes were negative for systems containing methanol and ethanol and positive for those containing 1-propanol and 1-butanol. It was also observed that an increase in temperature increased \(V_{{\text{ m}}}^{\text{E}}\) except for the ethyl levulinate + methanol system, where an increase in temperature decreased the excess molar volume values. However, when the length of the alkanol's carbon chain increased, the excess molar volume increased in the following order: methanol < ethanol < 1-propanol < 1-butanol.
All binary systems studied presented a negative \(\kappa_{{ \text{S}}}^{\text{E}}\). More negative excess isentropic compressibility values were observed at higher temperatures. The most negative excess isentropic compressibility values were observed for systems containing methanol and ethanol, with absolute following the order: methanol > ethanol > 1-propanol > 1-butanol.
With regard to the excess refractive indices, positive values were obtained for the systems containing methanol and ethanol. An increase in the temperature increased \(n_{{ \text{D}}}^{\text{E}}\) for the ethyl levulinate + methanol system; however, a decrease in \(n_{{\text{ D}}}^{\text{E}}\) was observed for the system containing ethanol. Conversely, a negative \(n_{{ \text{D}}}^{\text{E}}\) value was observed for systems containing 1-propanol and 1-butanol, and we noticed a more negative excess refractive index value for the system containing 1-butanol with increasing temperature. In both cases, an increase in temperature led to more negative \(n_{{ \text{D}}}^{\text{E}}\) values.
The excess molar enthalpy was positive for all systems studied. The maximum \(H_{{ \text{m}}}^{\text{E}}\) values for the four systems studied were obtained at the equimolar composition, x1 ≈ 0.5. An increase in temperature led to a higher excess molar enthalpy. When the length of the carbon chain of the alkanol increased, \(H_{{\text{ m}}}^{\text{E}}\) also increased, and the excess molar enthalpies increased in the following order: methanol < ethanol < 1-propanol < 1-butanol.
The sign and magnitude of the excess properties depend on both structural effects and molecular interactions. Some of them, such as \(V_{{ \text{m}}}^{\text{E}}\), \(\kappa_{{\text{ S}}}^{\text{E}}\), and \(n_{{ \text{D}}}^{\text{E}}\), are more closely related to structural effects, whereas excess molar enthalpy is related to molecular interactions.
The negative excess molar volume is owing to the presence of strong intermolecular interactions in the mixture and hydrogen bonding between the components compared to those existing in the pure components [36]. This implies ethyl levulinate can act as an acceptor in the hydrogen bond, and the alkanols can act as proton donors. However, structural effects due to differences in size and shape between the components may result in better molecular rearrangement between the two components [37, 38]. A positive excess molar volume implies the existence of less favourable intermolecular interactions caused by a decrease in proton donor power with the length of the carbon chain of the alkanol [39, 40] and a worse rearrangement between the components owing to the greater length of the carbon chains of 1-propanol and 1-butanol.
Negative excess isentropic compressibility values indicated that the mixture was less compressible and more compact than the components in their pure state. In our study, we obtained negative \(V_{{\text{ m}}}^{\text{E}}\) and \(\kappa_{{\text{ S}}}^{\text{E}}\) for the ethyl levulinate + methanol or ethanol systems and positive excess molar volume values and negative excess isentropic compressibility values for the other systems studied. Because the excess isentropic compressibilities are negative, the effect of the strong intermolecular interactions of the pure components, as well as the effect of the reduced size of the methanol and ethanol molecules, allows them to accommodate ethyl levulinate better, leading to mixtures containing methanol and ethanol having the lowest excess isentropic compressibility values.
According to Hirschfelder et al. [41], when the scattering forces increase, the speed of light decreases, and the refractive index increases. For systems containing methanol and ethanol, a clear reduction in the volume of the mixture was detected; thus, the dispersion increased, leading to positive excess refractive indices. For systems involving 1-propanol and 1-butanol, \(V_{{\text{ m}}}^{\text{E}}\) was positive, the volume of the mixture expanded, the dispersions decreased, and a negative \(n_{{ \text{D}}}^{\text{E}}\) was obtained.
The positive excess molar enthalpies were owing to the weakening of the strong interactions, dipole–dipole interactions, dispersion forces acting on ethyl levulinate, and the hydrogen bonds present in the alcohols. An exothermic effect was observed when the compounds were mixed owing to the formation of hydrogen bonds, particularly in the case of methanol, which has the highest proton donor capability among alcohols; therefore, the excess molar enthalpy is lower for the ethyl levulinate + methanol system.
We can now comment on the variations in excess properties of the binary systems alkyl levulinate + n-alkanols when substituting ethyl levulinate for methyl levulinate [3] at T = 298.15 K. Regarding excess molar volumes, different cases arise depending on the n-alkanol considered. For methanol, the values for the two alkyl levulinates were similar. For ethanol, the values obtained for the mixture with ethyl levulinate were slightly more negative up to the composition x1 ≈ 0.4, and then they were practically the same. Finally, for 1-propanol and 1-butanol, for which \(V_{{\text{ m}}}^{\text{E}}\) was positive, the excess molar volumes of the mixture containing ethyl levulinate were somewhat lower. Fewer negative values were obtained for excess isentropic compressibility, particularly for the mixture containing methanol. Different cases were also presented for the excess refractive indices. For methanol production, the values for ethyl levulinate were higher than those for methyl levulinate. For ethanol, the values presented by the mixture with ethyl levulinate were slightly more positive in the composition interval 0.3–0.7. Finally, for 1-propanol and 1-butanol, for which the \(n_{{ \text{D}}}^{\text{E}}\) was negative, the values for the mixture containing ethyl levulinate were less negative. In the case of excess molar enthalpies, lower values were obtained, particularly for the mixtures containing methanol.
Modelling
Density and excess molar enthalpy
The PC-SAFT EOS [7, 8] can be written in terms of the Helmholtz energy (\(A\)), which is the sum of the four terms given by Eq. (5):
where the superscripts id, hc, disp, and assoc are related to an ideal gas, hard-chain, dispersion forces, and association contribution, respectively.
From Eq. (5), the theoretical pressure can be obtained by equating it with the experimental pressure, and the liquid density can be calculated. More information on these equations can be reviewed in a study by Gross and Sadowski [7].
From Eq. (6), the excess molar enthalpy, \(H_{{\text{ m}}}^{\text{E}}\), can be obtained as follows:
where xi is the molar fraction of component i in the mixture, \(H_{\text{0,i}}^{\text{R}}\) (with i = 1 or 2) is the residual enthalpy of the pure component i, and HR is the residual enthalpy of the mixture, which is given by Eq. (7) [42]:
where \(a^{R} = \left( {\frac{{A - A^{\text{id}} }}{RT}} \right)\), is the dimensionless residual Helmholtz energy, R is the universal gas constant, T is the absolute temperature, and ρ is the molar density.
The PC-SAFT EoS parameters required for pure fluids are the number of segments m, segment diameter σ, depth of the pair potential energy ε, association energy of interaction εAB, and effective volume of interaction κAB between sites A and B on the molecule. The 1-alkanols were modelled with two association sites, that is, the 2 B scheme (one site in the hydrogen atom and one site in the oxygen atom), whereas the ethyl levulinate molecule was modelled with two negative sites [43]. In addition, because the alcohol molecule has both positive and negative sites, alcohols can self-associate. By contrast, ethyl levulinate does not self-associate. However, we must consider the cross-association between the alcohol and ethyl levulinate, which occurs between the positive and negative sites of different molecules [44].
Speed of sound
Using the SCFT [9], the speed of sound can be obtained without requiring adjustable parameters from Eq. (8):
where si represents the space-filling factor of component i in the mixture and c = 1600 m s−1. NA is Avogadro’s number, ρ is the molar density obtained using PC-SAFT, and di is the diameter of the molecule, which can be calculated using Eq. (9) [7, 8]:
Refractive index
The refractive indices of the mixtures, nD, can be predicted using the Laplace mixing rule [10] in Eq. (10):
where \({z}_{{\text{i}}}\) denotes the mass fraction. The PC-SAFT EoS was used to obtain the density. Notably, Eq. (10) was successfully used in the previous studies [45,46,47].
The pure-component parameters used in this study were obtained from previous studies [8, 43]. The absolute average relative deviations between the liquid densities obtained using the PC-SAFT EoS and the experimental values are listed in Table 5. According to the results in Table 5, PC-SAFT (with parameters obtained from the literature) correctly predicted the densities of the pure liquids (overall deviation = 0.98%).
The interaction parameters for the binary mixtures, kij, were obtained by minimising the function defined in Eq. (11):
The parameters obtained are listed in Table 6. From the approach used in this study, PC-SAFT was used as a fitting approach for density (overall deviation = 0.80%) and as a predictive approach for the speed of sound (overall deviation = 1.76%), refractive index (overall deviation = 0.11%), and excess molar enthalpy (overall deviation = 17.37%). According to these deviation values, the predictions for the speed of sound and refractive index are accurate; however, PC-SAFT is not capable of quantitatively representing the excess molar enthalpy, which can be explained by the fact that the binary parameter was not fitted from the phase equilibrium data. Figure 4 illustrates the modelling of the properties at T = 298.15 K for all mixtures. Clearly, the experimental density agreed with the density obtained using the PC-SAFT EoS. Furthermore, the results predicted for the refractive index obtained using the Laplace mixing rule were excellent. However, the predictions of the excess molar enthalpy and speed of sound (with the PC-SAFT EoS and SCFT) were in qualitative agreement with the experimental information.
Correlated densities and predicted speeds of sound, refractive indices, and excess molar enthalpies as a function of the mole fraction, x1, at T = 298.15 K and at p = 100 kPa for the binary mixtures ethyl levulinate (1) + alkanol (2): (black square) methanol; (red circle) ethanol; (green triangle) 1-propanol; (blue inverted triangle) 1-butanol; (coloured line) correlations or predictions
Conclusions
Thermophysical properties, densities, speed of sound, refractive indices, and excess molar enthalpies of four binary systems formed by ethyl levulinate + 1-alkanol (C1–C4) were reported at three temperatures (283.15, 298.15, and 313.15 K) and at pressure, p = 100 kPa. The calculated excess properties were correlated with the mole or volume fraction using a modified Redlich–Kister equation.
Excess molar volumes were negative for mixtures containing the two shorter alkanols and positive for the remaining systems. Excess isentropic compressibilities were negative for all mixtures. The excess refractive indices for these mixtures show opposite behaviour to the excess molar volumes. Furthermore, the excess molar enthalpies were positive for all mixtures.
To explain this thermophysical behaviour, it is necessary to consider the strong interactions that exist in pure compounds, both ethyl levulinate and 1-alkanols, as well as the possibility of hydrogen bonding between ethyl levulinate and 1-alkanols. The density and excess molar enthalpy of the binary mixtures were modelled using the equation of state. The overall deviations for all the pure fluids and binary mixtures were 0.98% and 0.80%, respectively. Finally, the overall deviation using the predictive approaches was 1.76% and 0.11% for the speed of sound and refractive index, respectively.
References
Malucelli LC, Guiotoku M, Maia CMBF, Carvalho Filho MAS. Optimal blending to improve the combustibility of biofuels: a waste-to-energy approach. J Therm Anal Calorim. 2022;147:5771–7. https://doi.org/10.1007/s10973-021-10955-4.
Darolia PJ, Malik S, Garg S, Chadha R, Sharma VK. Thermodynamic properties of liquid mixtures containing cyclic amines and isomeric picolines: excess molar volumes and excess isentropic compressibilities and excess heat capacities. J Therm Anal Calorim. 2022;147:3331–64. https://doi.org/10.1007/s10973-020-10507-2.
Abidi R, Artal M, Hichri M, Lafuente C. Experimental and modelled thermophysical behaviour of methyl levulinate(methyl 4-oxopentanoate) and n-alkanol systems. J Mol Liq. 2021;339: 116739. https://doi.org/10.1016/j.molliq.2021.116739.
Abidi R, Hichri M, Lafuente C, Hernández A. Surface tensions for binary mixtures of alkyl levulinate + alkanol: measurement and modeling. Int J Thermophys. 2023;44:33. https://doi.org/10.1007/s10765-022-03142-6.
Ramli NAS, Abdullah F. Study of density, surface tension, and refractive index of binary mixtures containing alkyl levulinate and n-alcohol from 298.15 to 323.15 K. J Chem Eng Data. 2021;66:1856–76. https://doi.org/10.1021/acs.jced.0c00694.
Majstorović DM, Petrović PI, Kijevčanin ML, Živković EM. Thermodynamic study of triacetin or ethyl levulinate and alcohol binary mixtures. J Chem Thermodyn. 2023;180: 107004. https://doi.org/10.1016/j.jct.2023.107004.
Gross J, Sadowski G. Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules. Ind Eng Chem Res. 2001;40:1244–60. https://doi.org/10.1021/ie0003887.
Gross J, Sadowski G. Application of the perturbed-chain SAFT equation of state to associating systems. Ind Eng Chem Res. 2002;41:5510–5. https://doi.org/10.1021/ie010954d.
Schaaffs W. The problem of a theoretical calculation of the velocity of sound for binary liquid mixtures. Acustica. 1975;33:272–6.
Laplace PS. Traité de mécanique celeste. Typ Crapelet. 1821;4:32.
Lafuente C, Artigas H, López MC, Royo FM, Urieta JS. Excess molar enthalpies for isomeric chlorobutanes with isomeric butanols. Phys Chem Liq. 2001;39:665–73. https://doi.org/10.1080/00319100108031683.
Monk P, Wadsö I. A flow micro reaction calorimeter. Acta Chem Scand. 1968;22:1842–52. https://doi.org/10.3891/acta.chem.scand.22-1842.
Marsh KN. Recommended reference materials for the realization of physico-chemical properties. Oxford: I.U.P.A.C., Blackwell Scientific Publications; 1987.
González EJ, Alonso L, Domínguez A. Physical properties of binary mixtures of the ionic liquid 1-methyl-3-octylimidazolium chloride with methanol, ethanol, and 1-propanol at T = (298.15, 313.15, and 328.15) K and at p = 0.1 MPa. J Chem Eng Data. 2006;51:1446–52. https://doi.org/10.1021/je060123k.
Alvarez VH, Mattedi S, Martín-Pastor M, Aznar M, Iglesias M. Thermophysical properties of binary mixtures of {ionic liquid 2-hydroxy ethylammonium acetate plus (water, methanol, or ethanol)}. J Chem Thermodyn. 2011;43:997–1010. https://doi.org/10.1016/j.jct.2011.01.014.
González B, Domínguez A, Tojo J. Dynamic viscosities, densities, and speed of sound and derived properties of the binary systems acetic acid with water, methanol, ethanol, ethyl acetate and methyl acetate at T = (293.15, 298.15, and 303.15) K at atmospheric pressure. J Chem Eng Data. 2004;49:1590–6. https://doi.org/10.1021/je0342825.
Singh S, Aznar M, Deenadayalu N. Densities, speeds of sound, and refractive indices for binary mixtures of 1-butyl-3-methylimidazolium methyl sulphate ionic liquid with alcohols at T = (298.15, 303.15, 308.15, and 313.15) K. J Chem Thermodyn. 2013;57:238–47. https://doi.org/10.1016/j.jct.2012.08.030.
Ortega J. Densities and refractive indices of pure alcohols as a function of temperature. J Chem Eng Data. 1982;27:312–7. https://doi.org/10.1021/je00029a024.
Rodríguez A, Canosa J, Tojo J. Physical properties of binary mixtures (dimethyl carbonate + alcohols) at several temperatures. J Chem Eng Data. 2001;46:1476–86. https://doi.org/10.1021/je0101193.
Gonçalves FAMM, Trindade AR, Costa CSMF, Bernardo JCS, Johnson I, Fonseca IMA, Ferreira AGM. PVT, viscosity, and surface tension of ethanol: new measurements and literature data evaluation. J Chem Thermodyn. 2010;42:1039–49. https://doi.org/10.1016/j.jct.2010.03.022.
Salinas R, Pla-Franco J, Lladosa E, Montón JB. Density, speed of sound, viscosity, and excess properties of binary mixtures formed by ethanol and bis(trifluorosulfonyl)imide-based ionic liquids. J Chem Eng Data. 2015;60:525–40. https://doi.org/10.1021/je500594z.
Coquelet C, Valtz A, Richon D, de la Fuente JC. Volumetric properties of the boldine + alcohol mixtures at atmospheric pressure from 283.15 to 333.15 K. A new method for the determination of the density of pure boldine. Fluid Phase Equilib. 2007;259:33–8. https://doi.org/10.1016/j.fluid.2007.04.030.
González EJ, González B, Macedo EA. Thermophysical properties of the pure ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide and its binary mixtures with alcohols. J Chem Eng Data. 2013;58:1440–8. https://doi.org/10.1021/je300384g.
Yang C, Lai H, Liu Z, Ma P. Density and viscosity of binary mixtures of diethyl carbonate with alcohols at (293.15 to 363.15) K and predictive results by UNIFACVISCO group contribution method. J Chem Eng Data. 2006;51:1345–51. https://doi.org/10.1021/je0600808.
Papari MM, Ghodrati H, Fadaei F, Sadeghi R, Behrouz S, Rad MNS, Moghadasi J. Volumetric and ultrasonic study of mixtures of 2-phenylethanol with 1-butanol, 2-butanol, and 2-methyl-1-butanol at T = (298.15–323.15) K and atmospheric pressure: measurement and prediction. J Mol Liq. 2013;180:121–8. https://doi.org/10.1016/j.molliq.2012.12.037.
Varfolomeev M, Zaitseva K, Rakipov I, Solomonov B, Marczak W. Speed of sound, density and related thermodynamic excess properties of binary mixtures of butan-2-one with C1–C4 n-alkanols and chloroform. J Chem Eng Data. 2014;59:4118–32. https://doi.org/10.1021/je5007604.
Iglesias M, Orge B, Tojo J. Refractive indices, densities and excess properties on mixing of the systems acetone + methanol + water and acetone + methanol + 1-butanol at 298.15 K. Fluid Phase Equilib. 1996;126:203–33. https://doi.org/10.1016/S0378-3812(96)03130-5.
Bajic D, Zivkovic E, Serbanovic S, Kijevcanin M. Volumetric and viscometric study of binary systems of ethyl butyrate with alcohols. J Chem Eng Data. 2014;59:3677–90. https://doi.org/10.1021/je5005752.
Nikitin ED, Popov AP, Bogatishcheva NS, Faizullin MZ. Critical temperatures and pressures, heat capacities, and thermal diffusivities of levulinic acid and four n-alkyl levulinates. J Chem Thermodyn. 2019;135:233–40. https://doi.org/10.1016/j.jct.2019.03.040.
Zabransky M, Ruzicka V, Majer V, Domalski ES. Heat capacity of liquids: volume 1-critical review and recommended values. J Phys Chem Ref Data Monogr. 1996;5:6.
Privat R, Jaubert J-N. Discussion around the paradigm of ideal mixtures with emphasison the definition of the property changes on mixing. Chem Eng Sci. 2012;83:319–33. https://doi.org/10.1016/j.ces.2012.07.030.
Benson G, Kiyohara O. Evaluation of excess isentropic compressibilities and isochoric capacities. J Chem Thermodyn. 1979;11:1061–4. https://doi.org/10.1016/0021-9614(79)90136-8.
Reis JCR, Lampreia IMS, Santos AFS, Moita MLCJ, Douheret G. Refractive index of liquid mixtures: theory and experiment. ChemPhysChem. 2010;11:3722–33. https://doi.org/10.1002/cphc.201000566.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8. https://doi.org/10.1021/ie50458a036.
Heintz A, Schmittecker B, Wagner D, Lichtenthaler R. Excess volumes of binary 1-alkanol/hexane mixtures at temperatures between 283.15 and 323.15 K. J Chem Eng Data. 1986;31:487–92. https://doi.org/10.1021/je00046a030.
Rafiee HR, Frouzesh F. The study of partial and excess molar volumes for binary mixtures of nitrobenzene and benzaldehyde with xylene isomers from T = (298.15 to 318.15) K and P = 0.087 MPa. J Adv Res. 2016;7:769–80. https://doi.org/10.1016/j.jare.2015.11.003.
Nikam PS, Jadhav SMTMC, Hasan M. Volumetric, viscometric and ultrasonic behavior of dimethylsulfoxide with normal alcohols (C1–C4) at 308.15 K. J Mol Liq. 1998;76:1–11. https://doi.org/10.1016/S0167-7322(97)00051-2.
Bhuiyan MMH, Uddin MH. Excess molar volumes and excess viscosities for mixtures of N,N-dimethylformamide with methanol, ethanol and 2-propanol at different temperatures. J Mol Liq. 2008;138:139–46. https://doi.org/10.1016/j.molliq.2007.07.006.
Marcus Y. Introduction to liquid state chemistry. New York: Wiley Interscience; 1977.
Mecke R. Infra-red spectra of hydroxylic compounds. Discuss Faraday Soc. 1950;9:161–77. https://doi.org/10.1039/df9500900161.
Hirschfelder JO, Curtiss CF, Bird RB. Molecular theory of gases and liquids. New York: Wiley; 1954.
Haarmann N, Enders S, Sadowski G. Heterosegmental modeling of long-chain molecules and related mixtures using PC-SAFT: 1. Polar compounds. Ind Eng Chem Res. 2018;58:2551–74. https://doi.org/10.1021/acs.iecr.8b03799.
Altuntepe E, Emel’yanenko VN, Forster-Rotgers M, Sadowski G, Verevkin SP, Held C. Thermodynamics of enzyme-catalyzed esterifications: II. Levulinic acid esterification with short-chain alcohols. Appl Microbiol Biotechnol. 2017;101:7509–21. https://doi.org/10.1007/s00253-017-8481-4.
Kleiner M, Sadowski G. Modeling of polar systems using PCP-SAFT: an approach to account for induced-association interactions. J Phys Chem C. 2007;111:15544–53. https://doi.org/10.1021/jp072640v.
Benkelfat-Seladji NL, Ouaar F, Hernández A, Muñoz-Rujas N, Bahadur I, Ahmed NCB, Montero E, Negadi L. Measurements and modeling of physicochemical properties of pure and binary mixtures containing 1,2-dimethoxyethane and some alcohols. J Chem Eng Data. 2021;66:3397–416. https://doi.org/10.1021/acs.jced.1c00131.
Benkelfat-Seladji NL, Ouaar F, Hernández A, Bahadur I, Muñoz-Rujas N, Singh SK, Montero E, Ahmed NCB, Negadi L. Density, speed of sound, refractive index of binary mixtures containing 2-ethoxyethanol and some alcohols: measurement and correlation. J Chem Thermodyn. 2022;170: 106762. https://doi.org/10.1016/j.jct.2022.106762.
Belhadj D, Negadi A, Hernández A, Mokbel I, Bahadur I, Negadi L. A study on mixing properties of binary mixtures of 1-hexene with alkoxyethanols at different temperatures. J Chem Thermodyn. 2022;172: 106820. https://doi.org/10.1016/j.jct.2022.106820.
Acknowledgements
The authors gratefully acknowledge, the financial assistance from Diputación General de Aragón and Fondo Social Europeo “Construyendo Europa desde Aragón” (E31_23R) and from ‘‘Ministère de l’Enseignement Supérieur et de la Recherche Scientifique de la Tunisie’’.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abidi, R., García-Gracia, M., Hernández, A. et al. Thermophysical properties of ethyl levulinate and n-alkanol systems. J Therm Anal Calorim 149, 3617–3629 (2024). https://doi.org/10.1007/s10973-024-12953-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-12953-8