Abstract
Vascular graft infection is still a life-threatening complication of reconstructive surgery. Among other options, application of cryopreserved homografts can eventuate favorable outcome, if graft replacement is necessary. The preparation and storage of these allografts need special infrastructure and deep subzero (− 80 °C) temperature. However, the longer storage time can lead to inferior results after implantation, based upon clinicians’ experiences. The goal of our investigation was to circumscribe the optimal storage time interval with differential scanning calorimetry (DSC) and histological evaluation, using porcine aorta. All samples were deep-freezed using − 80 °C. Cryopreservated grafts were melted after 4, 6, 12, 16, 20, 24, 28 and 52 weeks; then, DSC and different types of histology were performed. Light microscopy analysis showed significant changes in the connective tissue fibers’ structure from the 16th week; while, DSC measurements confirmed systematic decrease in the thermal stability from the same week during the follow-up period. Our investigation suggested that cryopreservation can lead to significant and increasing microstructural damage of the fibers following the 12th week; thus, the homograft implantation can result in higher success rate inside this timeframe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, the discovery and spread of the different types of artificial grafts, as well as the increasing number of vascular reconstructions, resulted in a higher complication rate at this field. Beside the typical vascular surgical complications, like reocclusion and bleeding, surgical site infections related to the synthetic graft implantation, demonstrated a growing amount. These vascular graft infections are still the most difficult challenge for vascular surgeons with a prominently high lethality and limb loss rate. The incidence of prosthetic graft infection ranges between 0.5 and 6%, depending on the operated region [1,2,3,4]. This rate is lower in case of endografts, and is higher in patients with critical limb-threatening ischemia [1, 4]. While, the synthetic graft sortiment became wider and wider from the simple PTFE and dacron grafts to their impregnated variants, the autologous great saphenous vein and deep vein remained the “gold standard” in cases of distal bypasses and also in septic vascular surgery [5,6,7,8,9,10,11,12]. However, about 20–45% of the patients has not suitable venous graft due to earlier varicectomy, venous insufficiency, thrombophlebitis or previous revascularization with vein graft [5,6,7,8, 12,13,14,15]. This percentage can reach 50%, when a second revascularization is needed with saphenous graft [5, 16]. Among other options, cryopreserved vascular allografts can be used with favorable outcome in both field, when autologous venous graft is not available [4, 7, 8]. The cadaver, cryopreserved homografts have some advantages, like high resistance against infections and reinfections, as well as superior patency in below-the-knee bypasses compared with artificial vascular grafts; however, there are some potential, unfavorable late complications of their usage, like aneurismatic degeneration or graft thrombosis [7, 8, 17,18,19]. The goal of cryopreservation is to preserve the functional characteristics of vascular tissues for virtually infinite time [20]. Thus, these grafts can be used for later vascular reconstructions [20, 21]. However, freezing the graft to − 80 °C, then thawing before implantation can lead to cell injury and structural changes in the tissue, which affect contractility and endothelial function of the vessel [20]. These potentially harmful consequences can decisively influence the graft quality, accordingly the long-term outcome of its utilization [19].
In our previous study, porcine aortic wall segments were examined with routine histology and differential scanning calorimetry to determine the effects of deep-freezing in the first 12 weeks. Our data demonstrated only minor changes of the vascular structure in the first 3 month, suggesting great applicability with this short storage time [19]. To determine the overtime applicability of these cryopreserved grafts more accurately, 1 year follow-up study was performed including routine and special histological examinations and differential scanning calorimetry.
Materials and methods
Surgical and laboratorial preparation
The present study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85–23, revised 1996) and was approved by the Local Institutional Committee on Animal Research, University of Pécs (BA02/2000-29/2001).
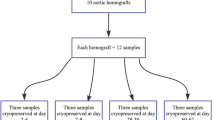
Segments of porcine aorta were freshly excised under sterile surgical conditions from euthanized Yorkshire pigs (mass from 23 to 27 kg) of other ongoing experiments, declared free of clinically evident diseases. After separation of the adhesive tissue, macroscopic examination was performed to exclude any pathology. Longitudinal aortic segments (about 2 cm in length with 5–7 mm diameter) were next washed in cold (4 °C), sterile Ringer Lactate solution (B. Braun Melsungen AG, Melsungen, Hessen, Germany) to remove residual traces of blood for 30 min. Thereafter, each aortic segment was placed separately in 50 mL preservative solution containing, sterilized Falcon tubes (Labsystem Kft., Budapest, Hungary) at room temperature for 20 min. The preservative solution contained: 1000 mL Ringer Lactate solution, 10 v/v% dimethyl sulfoxide (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany), 750 mg cefuroxime (GlaxoSmithKline plc., Brentford, Middlesex, United Kingdom) and 200 mg fluconazole (Fresenius Kabi Hungary Kft., Budapest, Hungary). Samples in Falcon tubes were next cryopreserved to − 80 °C using liquid nitrogen vapor (Messer Hungarogáz Kft., Budapest, Hungary) and then stored at this temperature in a deep freezer for further use. Cryopreserved aortic samples (5 samples in every timepoint) were thawed at room temperature in 4, 6, 12, 16, 20, 24, 28, 52 weeks and immediately following deep-freezing, thereafter histological evaluation and differential scanning calorimetry (DSC) analysis was performed.
Light microscopy evaluation
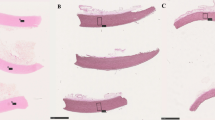
Aortic samples were fixed for 24 h in 10% neutral buffered formalin. Tissue blocks were embedded using paraffin, and 2–4 µm thick sections were sliced. Sections were stained applying Hematoxylin–eosin (HE), Masson trichrome (MT) and Orcein (O) histochemistry staining (Bio-Optica, Milano, Italy), using protocol recommended by the manufacturers. Each stained histological section, before and after DSC was compared and analyzed using Case Viewer software (3DHistec Ltd.), and was evaluated by two independent pathologists. Five samples were assessed in each timepoint, before and after DSC analysis.
DSC measurements
The thermal denaturation of the samples was investigated with a SETARAM Micro DSC-II calorimeter. Each analysis was performed within the range of 0 and 100 °C with a heating rate of 0.3 K min−1. Conventional Hastelloy batch vessels (Vmax = 1 mL) were applied for the experiment with an average sample mass of ~ 150 mg (range: 110–200 mg). Normal saline solution was used as a reference. The reference and sample vessels were equilibrated with a precision of ± 0.1 mg, so heat capacity correction between the sample and reference was not needed. The second scan of the denatured samples was used to make the baseline correction. The melting temperature of the samples (Tm) was defined as the peak of the heat transition curves (at this point the 50% of sample turns from native into denatured state). ΔT stands for the denaturation temperature range. The calorimetric enthalpy change (ΔHcal) was calculated from the area under the heat absorption curve with the SETARAM two points setting software. The sample mass in grams was used to normalize the ΔHcal values (in Jg−1 unit). We have finished our previous DSC investigation of freezing at the 12th week.
Results and discussion
Surgical site infections are possible complications of vascular reconstructions. The consequences can be lethal, if the inflammation spreads deep and affects the graft and/or the anastomosis, thus can lead to life-threatening bleeding and/or septicaemia. Treatment options depend on many factors, like the region and type of the infection, the type and virulence of the bacteria, the general condition of the patient, moreover the graft availability [1, 4]. To effectively treat graft infection, culture-directed, long-lasting antibiotical therapy and surgery are recommended [1, 4]. Accurate debridement, resection or removal of the infected graft, graft replacement, drainage and/or negative pressure wound therapy (NPWT) are the parts of surgical therapy [1, 4]. The gold standard for graft replacement is still the autologous graft, which can be the grand and small saphenous vein, superficial arm vein, deep vein (femoral) or desobliterated artery [1, 4, 7,8,9,10,11,12, 15, 17, 19]. If autologous graft is not available or the patient’s general condition is not enough fit for an extended surgery, the usage of another type of graft could be necessary. Synthetic grafts, especially their impregnated variants are generally useful for reconstructions, but with variable success rate regarding to reinfection [7, 22,23,24]. Application of cryopreserved vascular allografts or homografts is a highly recommended option with excellent results in infection elimination, however late, degenerative complications of their utilization are still under investigation by many researchers [4, 5, 7, 8, 15, 17,18,19]. There are some technical innovations to reduce the chance for graft impairment; however, deep-freezing and thawing of the vascular homograft can result in significant damage of the tissue [5, 20]. Besides this, the storage time at deep subzero temperature is an important factor, which can influence the late outcome of homograft utilization, but these experiences are not yet confirmed and based only upon clinicians’ observations [8, 15, 19]. In our investigation, we have made a genuine attempt to confirm the structural changes in the vascular tissue due to cryopreservation, for the purpose of improve the outcome of implantations and determine a more precise recommendation for optimal timing of utilization of homografts.
Differential scanning calorimetry (DSC) and 3 different types of histological analysis were performed to evaluate the deep-freezing caused structural alterations at different timepoints. Depending on our findings, we tried to determine the optimal storage time interval after cryopreservation, to improve long-term outcome of homograft implantation.
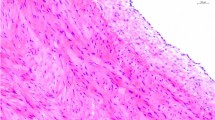
Hematoxylin–eosin staining was performed for routine histological examination, while Orcein staining demonstrated the structural changes of elastin fibers, as well as Masson trichrome histochemistry showed the collagen fiber alteration after cryopreservation, comparing the samples with freshly excised aortic wall (Fig. 1), in different time points.
Following deep-freezing we observed the damage of the endothelial layer in all samples at each timepoint. This effect was not dependent on storage time. The connective tissue fibers (elastin and collagen) did not show significant changes regarding their structure in the first 12 weeks. The runoff the fibers was decisively regular. After this timepoint, both collagen and elastin bundles became more and more irregular, undulatory and fragmentated, their staining pattern showed anomalism, and decreased eosinophilia. These changes were more pronounced near to the luminal surface, and intensified as the function of the storage time (Fig. 2).
Histological examinations were also performed after DSC measurements, showing similar alteration in fiber structure at different time points as mentioned above (Fig. 3). These findings were uniformed throughout all investigated samples.
We have finished our former investigation of freezing on aortic wall at 12th week [19]. It was extended in the recent study up to 52nd week. From the DSC scans (see Fig. 4) and the calculated thermal parameters (see Table 1) we can see the effect of cryoprotection in the function of time.
At the first sight can be seen two main denaturation ranges in all sample: in a lower and higher temperature range. The highest irreversible denaturation step is around 58 °C. This higher one is missing in the first cooling and the second heating/cooling steps. The lower Tm exhibited a significant decrease at 6th week (bold) and an increase in 24th and 52nd week (italic) compared to the control (see Table 1). ΔTL shows a significant decrease at 4,6,8 (8th is the lowest) and 28,52 weeks. This can refer to the stabilization of this thermal domain during the cryopreservation. Surpriseling the 12th week exhibits an increase, which is the sign of structure loosening. The calorimetric enthalpy in case of 6th as well as 12th–52nd weeks storing range was increased, reporting about thermally more stronger (rigid) structure as a consequence of cryoprotection. The second heating step exhibited significantly higher calorimetric enthalpy in the lower temperature range than the first one, which could be the sign the reversable structural reorganization of this thermal domain. The 16th and 20th weeks seem to be critical storage time. At 16th week the second cooling and in case of 20th the first and second cooling exhibited a biphasic reorganization, which cannot see neither before nor after this storage time.
TmH varied between 57.4 and 59.4 °C. We have observed a significant decrease compared to the control only at 2nd, 6th and 16th weeks. ΔTH decreased at 12th week, while increased (looser structure) at 16–20 and 28–52 weeks. The calorimetric enthalpy except of the 20th week was significantly smaller than the control one. It can show the storage time dependent effect during the frosening. We believe that this calorimetric enthalpy change is the best marker for monitoring structural damage caused by cryoprotective storage.
Conclusions
The implantation of cadaver, cryopreserved allografts is a widespread method in vascular surgery for distal bypasses or septic graft replacement, if eligible autologous vein graft is not available. The storage of these grafts needs special circumstances and infrastructure. In these tissue banks grafts are stored at − 80 °C to maintain their original structure; however, the longer storage time can harmfully influence the quality of the prosthesis, thus lead to higher complication rate. These alterations are usually non-visible, but histological examination together with DSC analysis can detect early structural changes, which can help us to appropriate graft selection.
In our study, light microscopy evaluation showed significant changes, progressive damage in the collagen and elastin fibers’ structure after the 12th week; while, DSC analysis demonstrated systematic decrease in thermal stability from the same week, during follow-up.
Based upon our findings, we suggest the utilization of these cryopreserved biological grafts in the first 12 weeks after deep-freezing. Grafts from this timeframe have more stable structure, which can prevent the patient from late complications.
This study could confirm the applicability of DSC in clinical aspects. Clinicians could make procedures more safely by detection of the cryoprotected vessel wall changes before implantation. With this method graft selection could be more optimal to avoid graft-material related complications.
Data availability
There are no additional available data to upload.
References
Mestres CA, Quintana E, Kopjar T, Ambrosioni J, Almela M, Fuster D, Ninot S, Miró JM, et al. Twenty-year experience with cryopreserved arterial allografts for vascular infections. Eur J Cardiothorac Surg. 2019;55:358–65.
Lorentzen JE, Nielsen OM, Arendrup H, Kimose HH, Bille S, Andersen J, et al. Vascular graft infection: an analysis of sixty-two graft infections in 2411 consecutively implanted synthetic vascular grafts. Surgery. 1985;98:1981–6.
Batt M, Magne J-L, Alric P, Muzj A, Ruotolo C, Ljungstrom K-G, et al. In situ revascularization with silver-coated polyester grafts to treat aortic infection: early and midterm results. J Vasc Surg. 2003;38:983–9.
Chakfé N, Diener H, Lejay A, Assadian O, Berard X, Caillon J, et al. Editor’s choice: European society for vascular surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339–84.
González-Gay M, López-Martinez R, Busto-Suárez S, Riedemann-Vistuba ME, Menéndez-Herrero MA, Álvarez-Marcos F, Alonso-Pérez M, Alonso-Arias R. Front Surg. 2020;7:616654.
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischaemia. Eur J Vasc Endovasc Surg. 2019;58:S1–109.
Fazekas G, Benkő L, Kasza G, Arató E, Sínay L, Jávor S, Nagy T, Hardi P, Kollár L, Jancsó G, Menyhei G. Histological and mechanical assessment of decellularized porcine biografts, and its biological evaluation following aortic implantation during mid-term follow-up. J Vasc Res. 2018;55:287–98.
Nagy Z, Oláh Z, Kókai J, Molnár AB, Laczkó Á, Szabó GV, Juhász V, Garbaisz D, Berczeli M, Sztupinszky Z, Szeberin Z. Role of the homograft bypass in extremity inferior’s reconstructions. Magy Seb. 2017;70(1):5–12.
Burger DH, Kappetein AP, Van Bockel JH, Breslau PJ. A prospective randomized trial comparing vein with polytetrafluoroethylene in above-knee femoropopliteal bypass grafting. J Vasc Surg. 2000;32:278–83.
Klinkert P, Post PN, Breslau PJ, Van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–62.
Berlakovich GA, Herbst F, Mittlböck M, Kretschmer G. The choice of material for above-knee femoropopliteal bypass. A 20-year experience. Arch Surg. 1994;129:297–302.
Hartranft CA, Noland S, Kulwicki A, Holden CR, Hartranft T. Cryopreserved saphenous vein graft in infrainguinal bypass. J Vasc Surg. 2014;60:1291–6.
Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001;15(3):241–78.
McPhee JT, Barshes NR, Ozaki CK, Nguyen LL, Belkin M. Optimal conduit chioce in the absence of single-segment great saphenous vein for below-knee popliteal bypass. J Vasc Surg. 2012;55:1008–14.
Hidi L, Komorowicz E, Kovács GI, Szeberin Z, Garbaisz D, Nikolova N, Tenekedjiev K, Szabó L, Kolev K, Sótonyi P. Cryopreservation moderates the thrombogenicity of arterial allografts during storage. PLoS ONE. 2021;16(7):e0255114.
Nguyen B-N, Neville RF, Abugideiri M, Amdur R, Sidawy AN. The effect of graft configuration on 30-day failure of infrapopliteal bypasses. J Vasc Surg. 2014;59:1003–8.
Töpel I, Uhl C, Ayx I, Steinbauer M. Xenografts in septic vascular surgery. Gefasschirurgie. 2016;21(Suppl2):55–8.
Fazekas G, Jancsó G, Lőrinczy D. DSC and histological analysis of decellularized porcine biograft. J Therm Anal Calorim. 2021;146:657–64.
Lőrinczy D, Fazekas G. DSC analysis of cryopreservation on the structure of porcine aortic biograft as a function of storage time. J Therm Anal Calorim. 2022;147:10411–7.
Müller-Schweinitzer E. Cryopreservation of vascular tissues. Organogenesis. 2009;5(97):104.
Wusteman MC, Pegg DE, Warwick RM. The banking of arterial allografts in the United Kingdom. A technical and clinical review. Cell Tissue Bank. 2000;1:295–301.
Young RM, Cherry KJ Jr, Davis PM, Gloviczki P, Bower TC, Panneton JM, Hallett JW Jr. The results of in situ prosthetic replacement for infected aortic grafts. Am J Surg. 1999;178(2):136–40.
Bandyk DF, Novotney ML, Johnson BL, Back MR, Roth SR. Use of rifampin soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res. 2001;95(1):44–9.
Bandyk DF, Novotney ML, Back MR, Johnson BL, Schmacht DC. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg. 2001;34(3):411–9.
Acknowledgements
This work was supported by CO-272 (OTKA) Grant (D.L.).
Funding
Open access funding provided by University of Pécs. Funding provided by University of Pecs.
Author information
Authors and Affiliations
Contributions
DL was involved in corresponding author; DSC contributed to experiments, data analysis, manuscript writing; LB helped in sample preparation and handling, histology; GF helped in sample preparation and handling, histology, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lőrinczy, D., Benkő, L. & Fazekas, G. Evaluation of the aortic wall structural alteration following cryopreservation in 1 year follow-up period. J Therm Anal Calorim 148, 13313–13320 (2023). https://doi.org/10.1007/s10973-023-12646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12646-8