Abstract
The utilization of cryopreserved human allografts is a recommended option in the septic vascular surgical field, if autologous graft is not available. These grafts are subjected to deep freezing and stored at − 80 °C until further utilization. The goal of our investigation was to determine the effect of cryopreservation on the structure of vessel wall as a function of storage time, using freshly excised porcine aortic grafts. The samples were subjected to deep freezing and cryopreservation at − 80 °C. Following immediately, 1, 2, 4, 6, 8 and 12 weeks after cryopreservation, differential scanning calorimetry (DSC) and routine histological examination were performed, comparing the structure of frozen grafts to fresh, native aortic wall. Light microscopy evaluation did not show significant changes in the structure of aortic wall at different time points; however, DSC measurements demonstrated a systematic decrease in the thermal stability up to the 6th week and then improvement and stabilization regarding this parameter till the 12th week. Our histological data suggest that cryopreservation causes only minor alteration in the microstructure of fibres in the first three months; thus, the utilization of deep-freeze biological grafts with this short storage time could give favourable outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of septic vascular prosthetic graft is still one of the most difficult challenges in vascular surgery. In most cases, complete healing can be assured only with successful graft replacement. The gold standard at this field is the utilization of autologous venous graft [1,2,3,4,5,6]. However, in about 20–40% of all patients, appropriate autologous vein graft is not available [1, 2, 6,7,8,9,10,11]. In these cases, impregnated (antibiotics, silver) prosthetic grafts, xenografts, biosynthetic grafts or cryopreserved vascular allografts (homograft) can be used [1, 8]. The utilization of homograft could be a favourable option [2, 8]. The advantage of these allografts is high resistance to infections and reinfections; however, there is a potential risk of graft degeneration [1, 2, 12]. Their production, storage and cryopreservation need special infrastructure. Regarding to this issue, there is no uniformed or preferred method in the literature. Different cryopreservation methodologies are in use [13]; however, according to the best of our knowledge, the effect of storage time on the microstructure of vascular tissue was not investigated previously. Fresh freezing of the graft to − 80 °C reduces the immunogenicity; nevertheless, it can cause structural changes within the extracellular matrix (ECM), which could influence the quality of the allograft, and consequently the long-term results of its utilization. Nagy et al. published the benefit in graft patency and limb salvage of homograft stored for less than 6 months following cryopreservation, but these findings based only upon their clinical experiences [2].
Thermal analysis (e.g. DSC) is a very useful method for monitoring early structural changes, as it is the only measurement method that can directly measure the change in the heat capacity (Cp) of the test sample. Biological samples are usually multi-subunit macromolecular systems in which the structural change induced by thermal excitation means a change in degrees of freedom, so Cp will also change. We have many years of experience in biological samples (cartilage, tendon, blood plasma, etc.) in DSC studies, where the thermal parameters of samples without visible structural change indicated non-physiological behaviour (cartilage [14,15,16], tendon [17, 18], muscle [19, 20], plasma [21,22,23,24]).
In the recent paper our aim is to analyse the effect of deep freezing and storage time on the structure of porcine aortic wall with differential scanning calorimetric (DSC) and histological examinations, to determine the optimal cryopreservation period for the routine clinical use of homograft.
Materials and methods
Surgical and laboratorial preparation
The present study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996) and was approved by the local institutional Committee on Animal Research, University of Pécs (BA02/2000-29/2001).
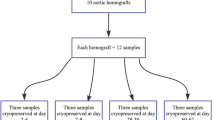
Aortic segments were freshly excised under sterile surgical conditions from anesthetized Yorkshire pigs (mass from 23 to 27 kg), declared free of clinically evident diseases. Following separation of the adhesive tissue, macroscopic examination was performed to exclude any pathology. Longitudinal aortic segments (about 2 cm in length with 5–7 mm diameter) were next washed in cold (4 °C), sterile Ringer Lactate solution (B. Braun Melsungen AG, Melsungen, Hessen, Germany) to remove residual traces of blood for 30 min. Thereafter, each aortic segment was placed separately in 50 mL preservative solution containing, sterilized Falcon tubes (Labsystem Kft., Budapest, Hungary) at room temperature for 20 min. The preservative solution contained: 1000 mL Ringer Lactate solution, 10v/v% dimethyl sulfoxide (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany), 750 mg cefuroxime (GlaxoSmithKline plc., Brentford, Middlesex, UK) and 200 mg fluconazole (Fresenius Kabi Hungary Kft., Budapest, Hungary). Samples in Falcon tubes were next cryopreserved to − 80 to 85 °C using liquid nitrogen vapour (Messer Hungarogáz Kft., Budapest, Hungary) and then stored at − 80 °C in a deep freezer for further use. Cryopreserved aortic samples (5 samples in every time point) were thawed at room temperature after 1, 2, 4, 6, 8, 12 weeks, and immediately following deep freezing, thereafter light microscopy evaluation and digital scanning calorimetry (DSC) analysis were performed. The results of thermal denaturation are presented in Table 1. Data are average of 5 measurements ± s.d. Statistical evaluation was made by t test at p < 0.05.
Light microscopy evaluation
Aortic samples were fixed for 24 h in 10% neutral buffered formalin, in support of routine histological examination. Tissue blocks were embedded using paraffin, and 2–4-µm-thick sections were sliced. Sections were stained applying haematoxylin–eosin (HE), analysed using Case Viewer software (3DHistec Ltd.), and were evaluated by two independent pathologists. Five samples were assessed in each time point, before and after DSC analysis.
DSC measurements
The thermal denaturation of the samples was investigated with a SETARAM Micro DSC-II calorimeter. Each analysis was performed within the range of 0 and 100 °C with a heating rate of 0.3 K min−1. Conventional Hastelloy batch vessels (Vmax = 1 mL) were applied for the experiment with an average sample mass of ~ 180 mg (range 110–400 mg). Normal saline solution was used as a reference. The reference and sample vessels were equilibrated with a precision of ± 0.1 mg, so heat capacity correction between the sample and reference was not needed. The second scan of the denatured samples was used to make the baseline correction. The melting temperature of the samples (Tm) was defined as the peak of the heat transition curves (at this point the 50% of sample turns from native into denatured state). ΔT stands for the denaturation temperature range. The calorimetric enthalpy change (ΔHcal) was calculated from the area under the heat absorption curve with the SETARAM two points setting software. The wet sample size in grams was used to normalize the ΔHcal values (in J g−1 unit).
Results and discussion
Septic complications play still an important role in vascular surgery, with high morbidity and mortality rate. In severe cases, the inflammation affects not only surgical site but also the area of reconstruction. The incidence of prosthetic graft infection ranges from 1 to 6% after implantation [8]. It depends on many factors, for example duration and region of the surgery, concomitant diseases, medications and presence of a trophic wound or ulcer [8]. If graft infection was developed, utilization of autologous graft can give the best solution [1,2,3,4,5,6, 8]. However, 20–40% of patients do not have appropriate vein, furthermore, the removal of veins prolongs the procedural time and increases the perioperative morbidity and, thus, this method is not suitable for all, especially for poor risk patients [1, 2, 6,7,8,9,10,11,12]. In these cases, the usage of a cryopreserved vascular allograft can serve as an alternative solution, with superb results in elimination of infection [1, 2, 8, 12]. The disadvantage of homograft utilization is late graft degeneration and aneurysm formation, intimal hyperplasia and graft thrombosis [1, 2]. Cryopreservation of the graft to − 80 °C with different methods [13] could damage the structure of the vessel, which effect could influence the quality of the homograft. Moreover, the storage time can also influence the biomechanical properties of the grafts and can lead to less favourable outcome after implantation. As far we know, there are only two publications in the literature regarding to this last topic. The first one (Nagy et al.) published only the clinical experience, as mentioned above [2], the second (Hidi et al.) found retained haemostatic and reduced thrombogenic potential of the homograft during the 6-month storage period [11].
In our investigations, differential scanning calorimetry (DSC) and routine histological analysis were performed to detect the changes in the microstructure of porcine aortic wall after cryopreservation, in a time-dependent fashion. DSC analysis is able to detect the structural alterations of macromolecules, before the defect of the tissue is evident [14,15,16,17,18,19,20,21,22,23,24]. Thus we tried to conclude the optimal storage time frame for homograft, in which a favourable outcome can be expected following utilization.
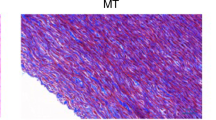
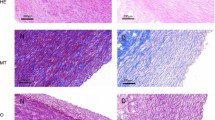
Haematoxylin–eosin (HE) staining was used for routine histological examination comparing fresh aortic wall with cryopreserved samples in different time points. After fast freezing of the aortic wall, the damage of the endothelial layer can be observed uniformly throughout all sections, independently of the storage time. The structure of collagen fibres shows minimal, not significant change in every samples. Apart from some fragmentation near the luminal surface, collagen bundles are regular, preserved their original structure, their staining pattern is regular and uniform, and decreased eosinophilia cannot be detected. These findings were also uniformed throughout all investigated samples, and duration of freezing does not influence this effect (see Figs. 1 and 2).
We have performed histological test after each denaturation too (see Fig. 3) with the same negligible differences (both cases change was only in collagen structure) as in case Fig. 2.
Looking on the denaturation scans (see Figs. 4–6) the first striking impression is that in case of porcine aortic samples there is a renaturation effect below 50 °C during the first heating, but above this range the tissue undergone to a full denaturation. In all cases the endotherm below 50 °C was significantly higher during the second heating than in case of first one. The calorimetric enthalpy (ΔHH) of the higher melting significantly decreased till the 6th-week cold preservation comparing with the control sample (see Table 1). The higher denaturation temperature range (ΔTH) diminished from the storage time of 4th week, but the melting point (TmH) decreased up to the 6th-week cold preservation. The main denaturation temperature (TmH) from the 8th week went back to the control value, but ΔHH increased compared with the 6th-week result (see Table 1). These data can prove the thermal consequence of structural change in porcine aorta evoked by the cold preservation up to 6th week. Another sign of it is the change in the run of the scans in the lower-temperature range during the 1st and 2nd heating, where we can distinguish at least two thermal domains (see Fig. 5.). The shape of the denaturation curves after 12 weeks’ of cryopreservation was similar to the control sample during both denaturation processes (see Fig. 6).
Conclusions
In order to improve the patient's condition in surgical practice, it is very important that the built-in devices (prostheses, grafts, etc.) used, during the operation, function reliably in the long run. This avoids corrective surgeries caused by infection or poor quality of the built-in material. Recognizing the importance of this in clinical practice, the so-called banks were created (e.g. bone, cartilage, tendon, etc., bank). The advantage of these is that the material required for corrective surgery is available even in unexpected cases. They are usually stored at − 80 °C, which in the case of long storage times can cause structural changes that compromise safe implantation.
These structural changes are not only detectable by the eye, but often by histological examination in cases where the use of this substance would later lead to complications.
Light microscopy evaluation did not show significant changes in the structure of aortic wall at different time points; however, DSC measurements demonstrated a systematic decrease in the thermal stability up to the 6th week and then improvement and stabilization regarding this parameter till the 12th week. Our histological data suggest that cryopreservation causes only minor alteration in the microstructure of fibres in the first three months; thus, the utilization of deep-freeze biological grafts with this short storage time could give favourable outcome.
In the recent paper we could prove the applicability of DSC to demonstrate the early (e.g. by histology not detectable) changes in aortic samples undergone to cryoprotection. This allows all surgeons to choose more safely from frozen grafts, avoiding postoperative intervention following possible injury. An additional follow-up study of at least one year is required to more accurately determine the applicability of the grafts over time, and other (e.g. biochemical) technique should be involved to clarify the reason of the improvement of thermal stability of frozen samples.
Availability of data and material
There are no additional available data to upload.
References
Fazekas G, Benkő L, Kasza G, Arató E, Sínay L, Jávor S, Nagy T, Hardi P, Kollár L, Jancsó G, Menyhei G. Histological and mechanical assessment of decellularized porcine biografts, and its biological evaluation following aortic implantation during mid-term follow-up. J Vasc Res. 2018;55:287–98.
Nagy Z, Oláh Z, Kókai J, Molnár AB, Laczkó Á, Szabó GV, Juhász V, Garbaisz D, Berczeli M, Sztupinszky Z, Szeberin Z. Role of the homograft bypass in extremity inferior’s reconstructions. Magy Seb. 2017;70(1):5–12.
Burger DH, Kappetein AP, Van Bockel JH, Breslau PJ. A prospective randomized trial comparing vein with polytetrafluoroethylene in above-knee femoropopliteal bypass grafting. J Vasc Surg. 2000;32:278–83.
Klinkert P, Post PN, Breslau PJ, Van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–62.
Berlakovich GA, Herbst F, Mittlböck M, Kretschmer G. The choice of material for above-knee femoropopliteal bypass. A 20-year experience. Arch Surg. 1994;129:297–302.
Hartranft CA, Noland S, Kulwicki A, Holden CR, Hartranft T. Cryopreserved saphenous vein graft in infrainguinal bypass. J Vasc Surg. 2014;60:1291–6.
Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001;15(3):241–78.
Chakfé N, Diener H, Lejay A, Assadian O, Berard X, Caillon J, et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339–84.
McPhee JT, Barshes NR, Ozaki CK, Nguyen LL, Belkin M. Optimal conduit chioce in the absence of single-segment great saphenous vein for below-knee popliteal bypass. J Vasc Surg. 2012;55:1008–14.
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischaemia. Eur J Vasc Endovasc Surg. 2019;58:S1–109.
Hidi L, Komorowicz E, Kovács GI, Szeberin Z, Garbaisz D, Nikolova N, Tenekedjiev K, Szabó L, Kolev K, Sótonyi P. Cryopreservation moderates the thrombogenicity of arterial allografts during storage. PLoS ONE. 2021;16(7):e0255114.
Töpel I, Uhl C, Ayx I, Steinbauer M. Xenografts in septic vascular surgery. Gefasschirur-gie. 2016;21(Suppl2):S55–8.
Müller-Schweinitzer E. Cryopreservation of vascular tissues. Organogenesis. 2009;5:97–104.
Szabó I, Patczai B, Lőrinczy D. Effects of long-term deep freezing on human femoral cartilage: differential scanning calorimetric (DSC) analysis and histopathological evaluations. J Thermal Anal Calorim. 2021. https://doi.org/10.1007/s10973-021-11070-0.
Naumov I, Wiegand N, Patczai B, Vámhidy L, Lőrinczy D. Differential scanning calorimetric examination of the human hyaline cartilage of the femoral head after femoral neck fracture. J Thermal Anal Calorim. 2012;108:59–65.
Sillinger T, Lőrinczy D, Kocsis B, Kereskay L, Nöt LG, Wiegand N. Differential scanning calorimetric measurement of cartilage destruction caused by Gram-negative septic arthritis. J Thermal Anal Calorim. 2014;116:747–52.
Wiegand N, Vámhidy L, Kereskai L, Lőrinczy D. Differential scanning calorimetric examination of the ruptured Achilles tendon in human. Thermochim Acta. 2010;498:7–10.
Wiegand N, Naumov I, Vámhidy L, Kereskai D, Lőrinczy LG. Comparative calorimetric analysis of 13 different types of human healthy and pathologic collagen tissues. Thermochim Acta. 2013;568:171–4.
Szabó I, Bognár G, Kereskai L, Szász K, Lőrinczy D. Differential scanning calorimetric and histological examinations of the long head of the biceps in cadavers. J Thermal Anal Calorim. 2007;88:343–9.
Wiegand N, Vámhidy L, Patczai B, Dömse E, Kereskai L, Lőrinczy D. Differential scanning calorimetric examination of the human skeletal muscle in a compartment syndrome of the lower extremities. J Thermal Anal Calorim. 2009;98:177–82.
Zapf I, Fekecs T, Ferencz A, Tizedes G, Pavlovics G, Kálmán E, Lőrinczy D. DSC analysis of human plasma in breast cancer patients. Thermochim Acta. 2011;524:88–91.
Mehdi M, Fekecs T, Zapf I, Ferencz A, Lőrinczy D. Differential scanning calorimetry (DSC) analysis of human plasma in different psoriasis stages. J Thermal Anal Calorim. 2013;111:1801–4.
Ferencz A, Zapf I, Lőrinczy D. Harmful effect of neoadjuvant chemotherapy monitoring by DSC on breast cancer patients’ blood plasma. J Thermal Anal Calorim. 2016;126:55–69.
Farkas P, Könczöl F, Lőrinczy D. Examination of the blood plasma and red blood cells in cyclophosphamide monotherapy with DSC in animal models. J Thermal Anal Calorim. 2017;127:1239–43.
Acknowledgements
This work was supported by CO-272 (OTKA) grant (D.L.).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
GF helped in sample preparation and handling, histology, manuscript writing. DL was involved in corresponding author, DSC experiments, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lőrinczy, D., Fazekas, G. DSC analysis of cryopreservation on the structure of porcine aortic biograft as a function of storage time. J Therm Anal Calorim 147, 10411–10417 (2022). https://doi.org/10.1007/s10973-022-11290-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11290-y